(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX, was host to a non-invasive bladder cancer moderated poster session. Dr. Amanda Myers presented the results of a single center analysis evaluating the efficacy of a repeat BCG challenge in patients with “BCG unresponsive” non-muscle invasive bladder cancer (NMIBC).
Intravesical BCG currently remains the standard-of-care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk NMIBC due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 However, despite adequate BCG treatment, defined as receipt of at least five doses of the initial six-dose induction course and at least 2/3 maintenance doses or at least 2/6 doses of the second induction course, up to 50% of such patients will develop a BCG-refractory, relapsing, or failure state.4
In 2018, the FDA issued a guidance document to facilitate the development of drugs and biologics in this disease space. In an attempt to standardize disease definitions and facilitate clinical trial design, BCG-unresponsive NMIBC was defined as follows:5
- Persistent or recurrent CIS within 12 months of adequate BCG therapy
- Recurrent high-grade Ta/T1 disease within six months of completion of adequate BCG therapy
- High-grade T1 disease detected on the first evaluation following an induction BCG course
Given the emergence of novel approved agents in the BCG-unresponsive state, re-challenging patients with additional courses of BCG has fallen out of favor in clinical practice. However, given the limited options, as well as costs associated with certain treatment modalities, such an approach remains an option in select circumstances. The objective of this analysis was to report the response rates to additional (rescue) BCG in patients with BCG-unresponsive NMIBC.
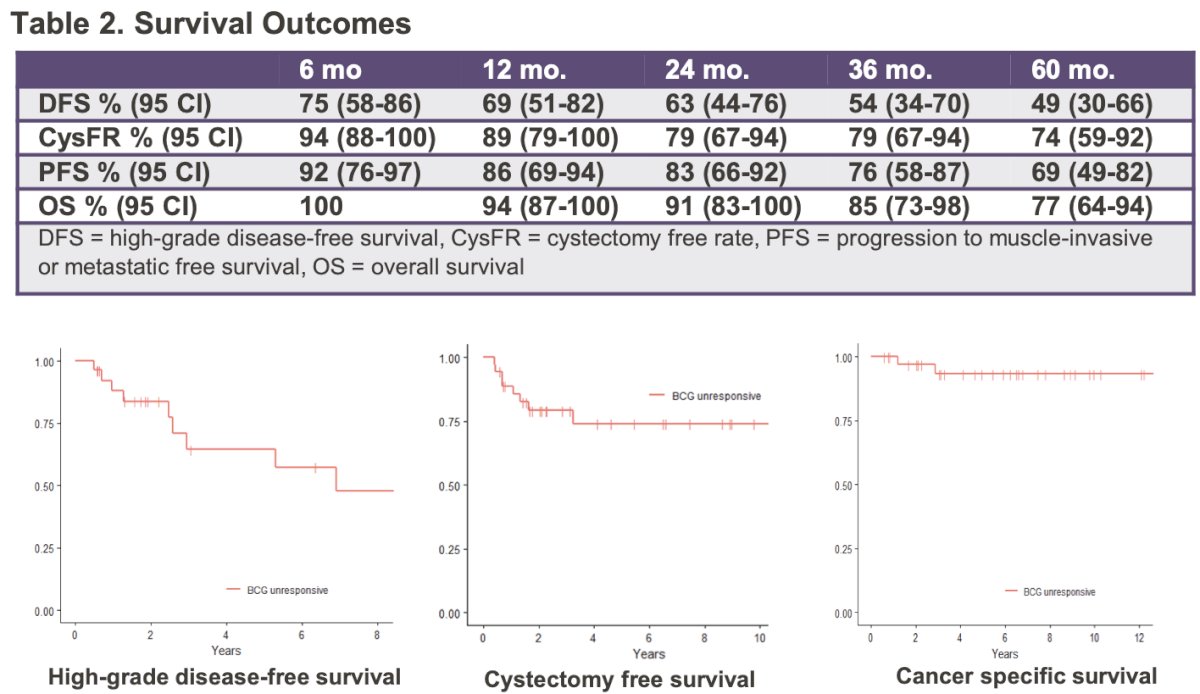
An IRB approved review of consecutive patients diagnosed with NMIBC between January 2000 and September 2021 was performed to identify patients who met the BCG unresponsive criteria. The investigators analyzed the outcomes of patients who received rescue BCG as primary therapy. The primary outcome evaluated was high-grade disease-free survival (DFS). The Kaplan- Meier (KM) method was used to estimate DFS, cystectomy free survival (CFS), progression to muscle-invasive or metastatic free survival (PFS) and OS.
The baseline patient characteristics are summarized below (n=35). The median patient age in the cohort was 69 years. Almost 50% of patients had CIS disease present.

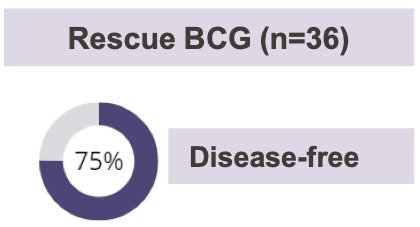
The median follow-up was 5.9 years. 69% of patients received repeat induction BCG, and 31% received maintenance BCG. 75% of patients had no disease following rescue BCG, and 96% of responders were receiving ongoing maintenance BCG at the time of analysis.
The median disease-free survival was 75 months (95% CI: 32 – NR). 25% of patients underwent a cystectomy at a median of 13 months.

At 12 months, survival outcomes were as follows:
- DFS: 68%
- Cystectomy-free survival: 89%
- PFS: 91%
- OS: 94%
At three years, the corresponding survival outcomes were as follows:
- DFS: 48%
- Cystectomy-free survival: 79%
- PFS: 76%
- OS: 85%
The swimmer plot below summarizes individual patient outcomes:

The investigators concluded that:
- Rescue BCG can have durable efficacy in appropriately selected patients with “BCG unresponsive” NMIBC
- 27 (75%) patients were disease free after rescue BCG with a median disease-free duration of 75 months.
- They argued that these findings challenge current paradigms which recommend against further BCG therapy and underscore the need for randomized controlled trials in this setting
Presented by: Amanda Myers, MD, Society of Urologic Oncology Fellow, Department of Surgery, The University of Texas, MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on May 3, 2024.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- Bacillus Calmette-Guérin-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bacillus-calmette-guerin-unresponsive-nonmuscle-invasive-bladder-cancer-developing-drugs-and. Accessed 22 Aug 2022. Accessed on May 3, 2024.


