(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to a non-invasive bladder cancer moderated poster session. Dr. Alessio Finocchiaro presented confirmatory results of the HuNIRe trial of replacing re-staging TURBT with cystoscopy in patients with high-grade T1 non-muscle invasive bladder cancer (NMIBC).
A re-staging (i.e., second resection) TURBT is currently recommended by numerous guidelines for all T1 NMIBCs, irrespective of the completeness of the initial resection and/or of the presence of detrusor muscle in the pathological specimen. However, the true impact of a re-staging TURBT on oncologic outcomes remains a subject of debate.
The study investigators have previously reported on the feasibility and safety of replacing re-staging TURBT with urine cytology and in-office flexible cystoscopy in select T1 NMIBC patients. The aim of this study is to confirm these findings and present the associated oncological outcomes.
This is an ongoing prospective multicenter trial enrolling patients diagnosed with HG T1 bladder cancer from five Italian hospitals. In case of complete tumor resection at initial TUR, HG T1 patients underwent a strict follow-up consisting of urine cytology at 4–6 weeks and an in-office flexible white-light and narrow-banding cystoscopy at 6–8 weeks. In case of a positive urine cytology or evidence of recurrence cystoscopically, re-staging TURBT was performed within two weeks. Otherwise, patients started the BCG induction course without any additional resection. Patients with a macroscopic incomplete initial resection or did not have detrusor muscle sampled underwent standard re-staging TURBT within 40 days.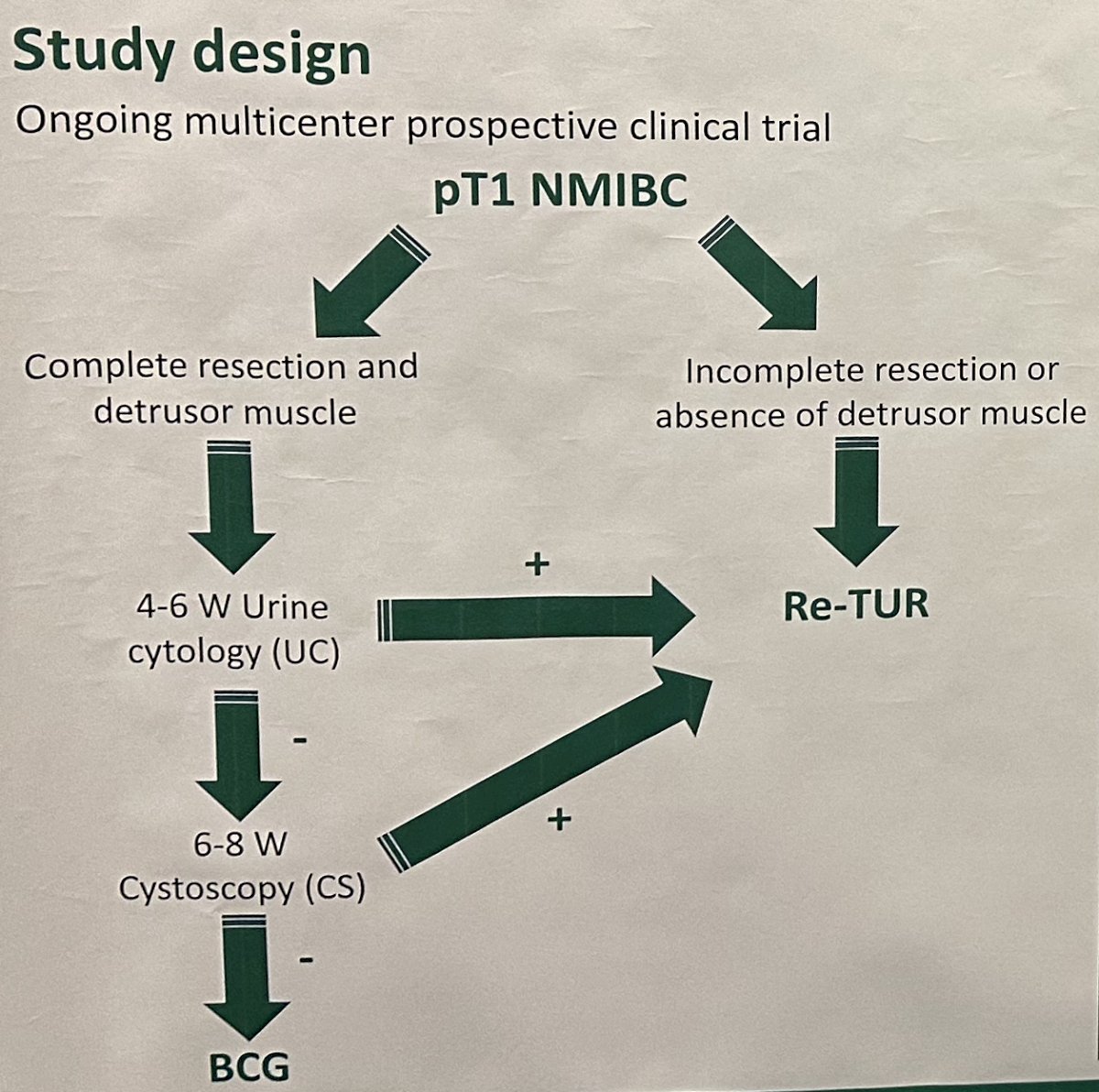
Overall, 89 patients were enrolled between May 2020 and January 2023, of whom 70 were deemed trial eligible. The median age was 76 years (IQR: 68–80). A total of 47 patients (68%) had a negative urine cytology and cystoscopy after TUR and thus proceed to BCG directly. Conversely, 23 (32%) patients had either a positive urine cytology or cystoscopy and underwent a re-staging TURBT. The presence of CIS at diagnosis was associated with increased odds of requiring a re-staging TURBT (HR: 5.3, 95% CI: 0.98-28.8, p=0.05).
At a median follow-up of 17.5 months (IQR: 13.6–24), 18 patients recurred and 2 progressed. In the overall cohort, the 2-year recurrence-free and progression-free survivals were 64% and 97%, respectively. There were no significant differences in recurrence-free survival between patients who underwent and avoided re-staging TURBTs (p=0.8).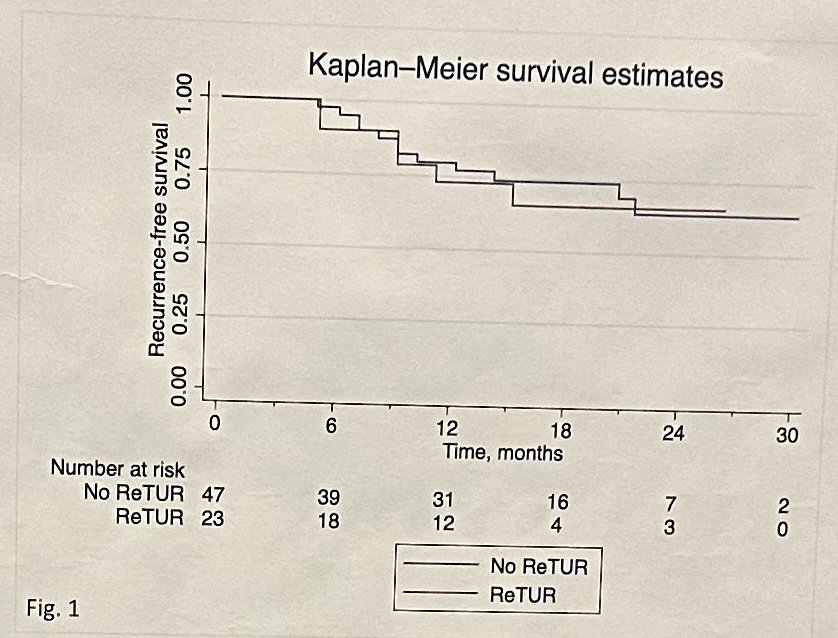
These ongoing results confirm that avoiding immediate re-staging TURBT in HG T1 patients and selectively offering it promptly only to those with a positive cystoscopy or urine cytology is not associated with worse cancer outcomes. Longer follow-up and larger sample sizes are necessary to validate these results.
Presented by: Alessio Finocchiaro, MD, Humanitas Research Hospital, Milan, Italy
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024


