(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a plenary session, and a presentation by Dr. Joseph Jacob discussing results from the SunRISe-1 study assessing TAR-200 in patients with BCG-unresponsive high risk non-muscle invasive bladder cancer.
Standard of care for BCG-unresponsive high-risk non-muscle invasive bladder cancer is radical cystectomy, however, this is a life-changing operation associated with considerable morbidity and impact on quality of life and a 90-day mortality risk of up to 8%. Moreover, many patients are unwilling to undergo radical cystectomy. There are limited treatment options available to treat BCG unresponsive high risk non-muscle invasive bladder cancer CIS, with 12-month complete response rates of 19% with pembrolizumab, 25% with atezolizumab, and 23% with nadofaragene firadenovec. TAR-200 is a novel targeted releasing system designed for sustained, local delivery of gemcitabine in the bladder, which is placed in the bladder using a urinary placement catheter in a 2-3 minute office procedure.
SunRISe-1 is an ongoing open-label phase 2b study among patients with BCG unresponsive NMIBC and the following trial design: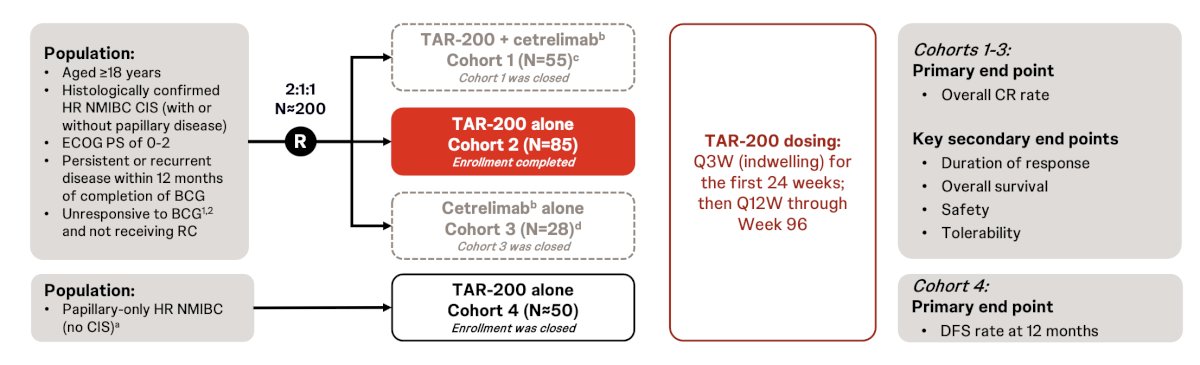
The primary endpoint is the overall complete response rate, and key secondary endpoints include: (i) duration of response, (ii) overall survival, (iii) safety, and (iv) tolerability. Response is determined by quarterly cystoscopy, quarterly central cytology, and central pathology at weeks 24 and 48 as clinically indicated. The study protocol did not allow re-induction for non-responders, consistent with US FDA guidance. At the 2024 AUA annual meeting, Dr. Jacob and colleagues presented updated results of the TAR-200 monotherapy cohort (Cohort 2).
Among 85 patients in cohort 2, the median age was 71 years (IQR 40-88), 80% were male, 72.9% were White, 67.1% were CIS only, the median total number of prior BCG doses was 12 (range 7-42):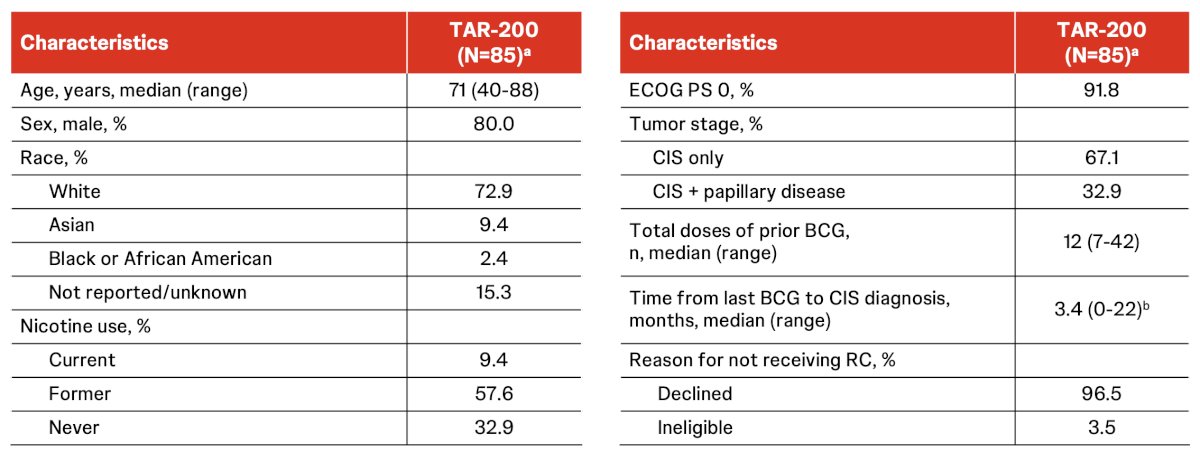
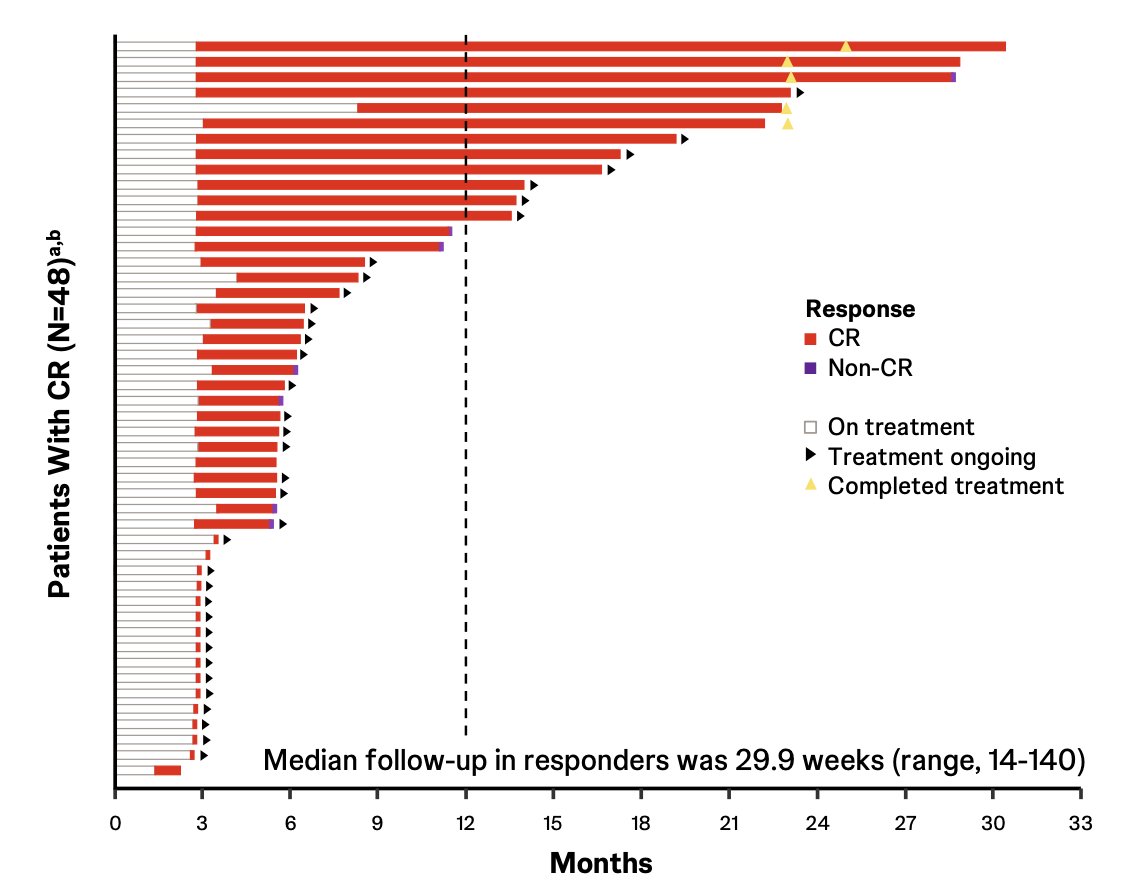
There was an 82.8% complete response rate via central review and an 86.2% complete response rate via investigator assessment, with the complete response rates at 6 and 12 months estimated to be 75.7% and 61.9%, respectively. Onset of response was rapid, with 47 of 48 (98%) complete responses achieved at the first disease assessment at week 12: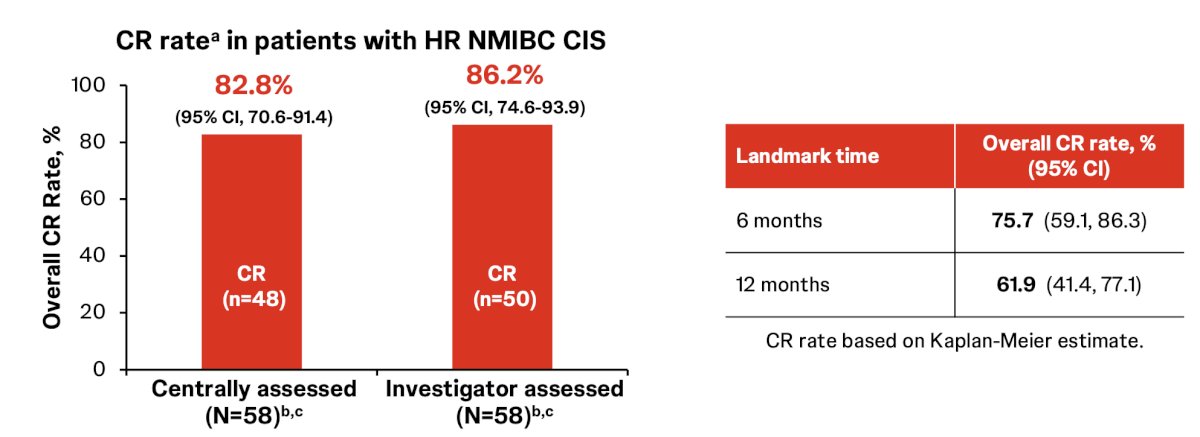
TAR-200 was also associated with a durable response, with 41 of 48 (85%) responses ongoing at clinical cutoff, with 4 of 5 (80%) who completed 2-year treatment remaining in response. None of the responders progressed to MIBC or metastatic disease, and only 1 of 48 (2.1%) responders underwent a radical cystectomy. The Kaplan-Meier estimates for the duration of response:
- 6-month duration of response rate: 87.0% (95% CI, 69.0-94.9)
- 12-month duration of response rate: 74.6% (95% CI, 49.8-88.4)
- 18-month duration of response rate: 74.6% (95% CI, 49.8-88.4)
TAR-200 monotherapy complete response rates were consistent across patient subgroups: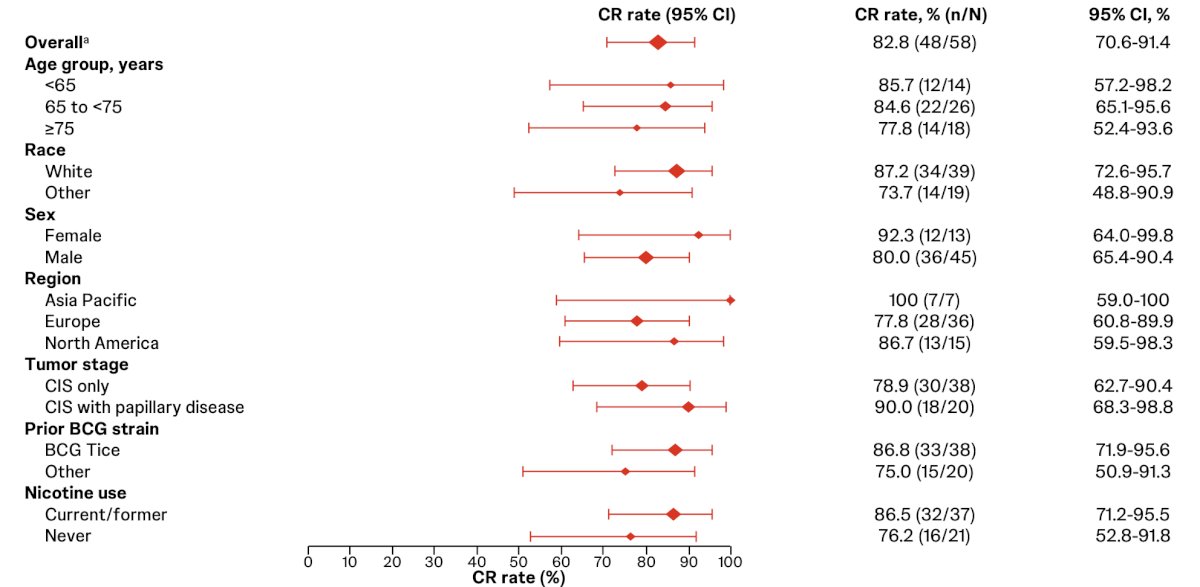
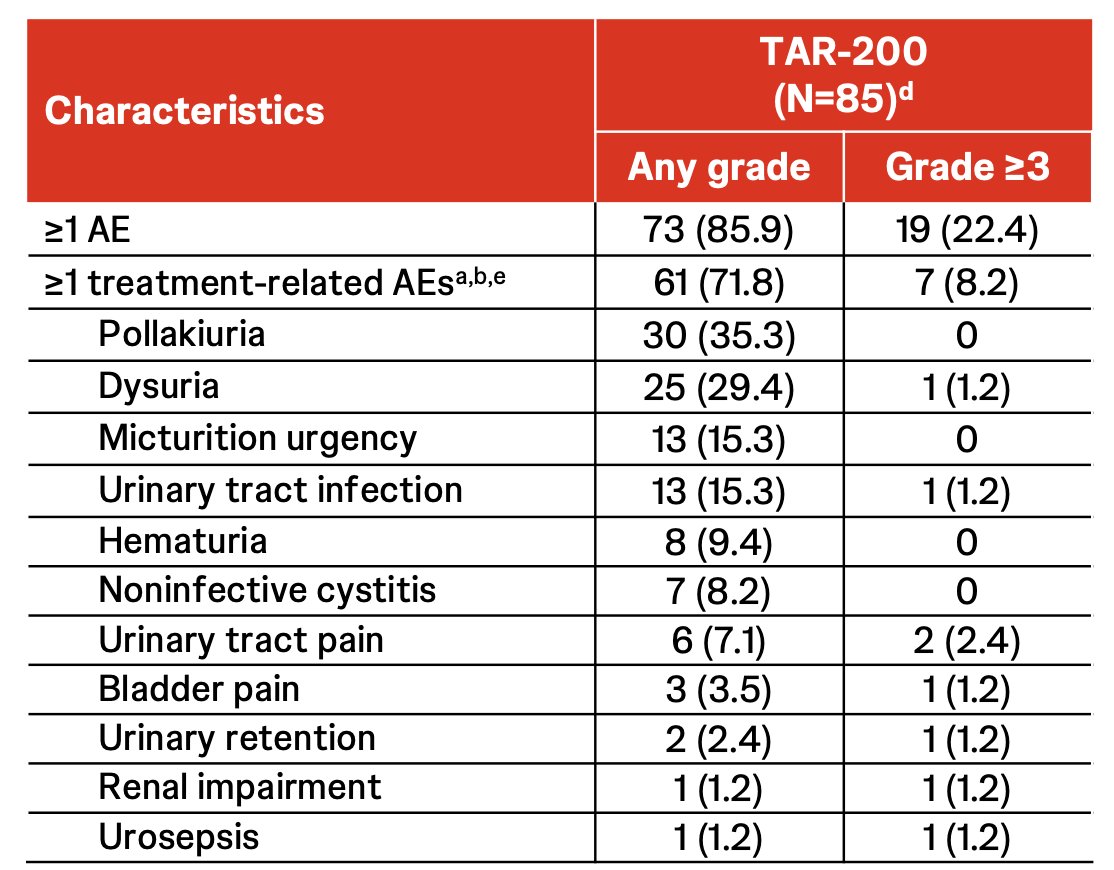
Most adverse events were grade 1 or 2, with onset within 12 weeks of treatment initiation and most resolving within 2 weeks:
- 61 patients (71.8%) had ≥1 treatment-related adverse events
- 4 patients (4.7%) had ≥1 serious treatment-related adverse events
- 7 patients (8.2%) had grade 3 treatment-related adverse events
- Few patients (n = 4; 4.7%) discontinued treatment due to adverse events
- No treatment-related deaths were reported
Dr. Jacob concluded his presentation discussing results from the SunRISe-1 study with the following take-home messages:
- TAR-200 monotherapy provides a high cure rate of complete response with rapid onset of response in patients with BCG-unresponsive high risk non-muscle invasive bladder cancer
- 98% of complete responses were achieved at 12 weeks
- 82.8% complete response rate via central review and 86.2% complete response rate via investigator assessment
- The complete response rates at 6 and 12 months were estimated to be 75.7% and 61.9%, respectively
- TAR-200 responses were durable
- 85% of responders are ongoing, with a median follow-up of 30 weeks
- 1-year duration of response rate was 74.6% (95% CI, 49.8-88.4) based on Kaplan–Meier estimate
- 5 patients have completed 2 years of treatment; 4 of these patients remain in complete response
- None of the responders progressed to muscle-invasive or metastatic disease
- 1 of 48 (2.1%) responders have undergone radical cystectomy
- Most adverse events were grade 1 or 2, and there were few treatment discontinuations secondary to adverse events
- TAR-200 has been granted FDA Breakthrough Therapy designation
Presented by: Joseph Jacob, MD, MCR, Urologist, Upstate Medical University, Syracuse, NY
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 - Mon, May 6, 2024.


