(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to a non-invasive bladder cancer moderated poster session. Dr. Reuben Ben David presented the results of a study comparing BCG to gemcitabine + docetaxel in intermediate-risk, non-muscle invasive bladder cancer (NMIBC) with mostly high-grade Ta disease.
Sequential gemcitabine plus docetaxel intravesical chemotherapy has emerged as an alternative treatment option for BCG naïve patients, particularly given the BCG shortages that have been experienced over the past few years. In this study, Dr. Ben David and colleagues sought to compare recurrence-free survival among patients receiving BCG versus gemcitabine plus docetaxel instillations for intermediate-risk NMIBC.
They conducted a retrospective analysis of consecutive patients treated with gemcitabine + docetaxel or BCG intravesical instillations for NMIBC between 2012 and 2023. Risk stratification was performed per the AUA risk stratification system. Patients with upper tract urothelial carcinoma were excluded. The Kaplan-Meier method was used to assess recurrence-free survival (any or high-grade).
This study included a total of 118 patients, with a median follow-up of 31 months (IQR: 16–59). The median patient age was 70 years, and 21,2% were female. This cohort of AUA-defined intermediate-risk patients included 66 (56%) patients with HG Ta disease and 52 (44%) with LG Ta disease. 77 patients received BCG, whereas 41 received gemcitabine + docetaxel.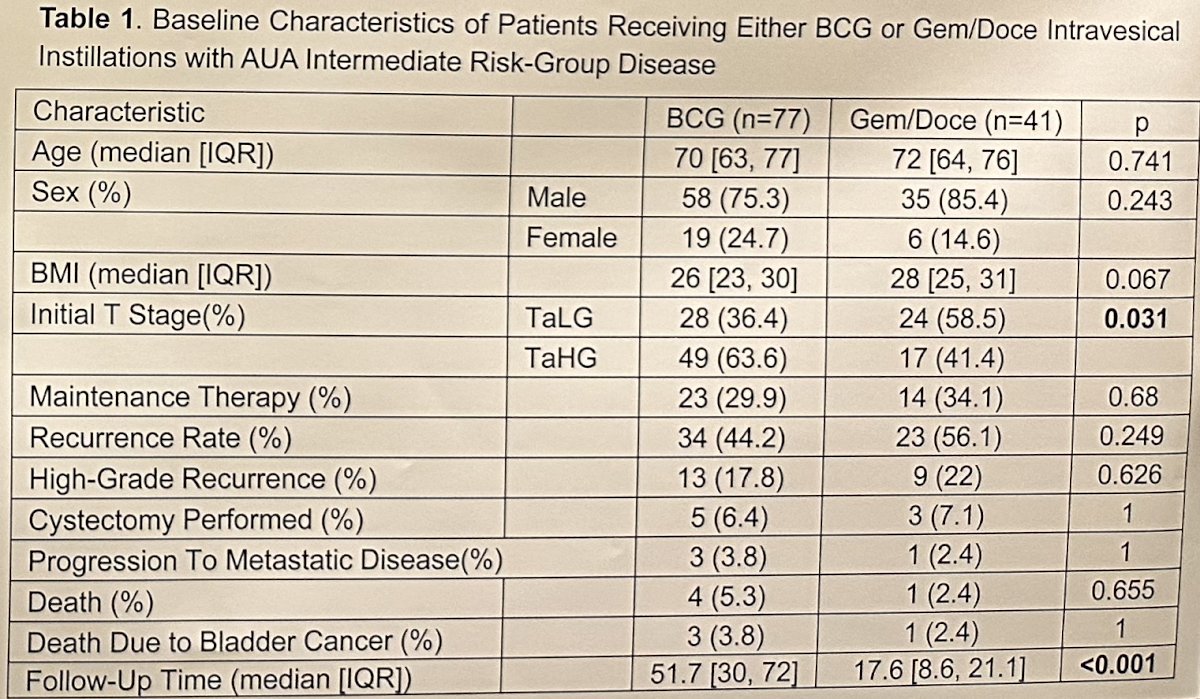
There were comparatively more patients with HG Ta disease in the BCG group (64% versus 43%; p=0.031). Maintenance therapy was administered in 30% of patients in the BCG group versus 34% in the gemcitabine/docetaxel group.
In the entire cohort, any grade and high-grade recurrences were observed in 48.3% (n=57) and 19.5% (n=23) of the patients, respectively. Patients in the gemcitabine/docetaxel group had significantly worse any-grade recurrence-free survival, compared to BCG-treated patients (log-rank test p=0.009). The following recurrence-free survival rates were observed for gemcitabine/docetaxel versus BCG:
- 12 months: 67% versus 70%
- 18 months: 52% versus 68%
- 24 months: 35% versus 62%
High-grade recurrence-free survival rates were similar between the two groups (log-rank p=0.36). 
Progression to metastatic disease occurred in 2.4% of patients in the gemcitabine/docetaxel group, compared to 3.9% of patients in the BCG group.
In a cohort of intermediate-risk patients consisting mainly of HG Ta disease, use of intravesical gemcitabine + docetaxel was associated with worse any-grade recurrence-free survival, compared to BCG. No differences were noted for high-grade recurrence-free survival. Dr. Ben David noted that previously reported studies mainly compared patients with primary or recurrent LG Ta disease. These findings should be considered when tailoring the treatment for HG Ta patients. Prospective trials with a larger cohort are necessary to validate these results.
Presented by: Reuben Ben David, MD, Society of Urologic Oncology (SUO) Fellow, Icahn School of Medicine, Mount Sinai Hospital, New York, NY
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024


