(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a non-invasive bladder cancer moderated poster session. Dr. Andrew Gabrielson presented the results of a phase II trial of intravesical gemcitabine + docetaxel in the treatment of BCG-naïve non-muscle invasive bladder cancer (NMIBC).
Combination intravesical gemcitabine plus docetaxel has demonstrated benefit for bacillus Calmette-Guerin (BCG) unresponsive NMIBC in retrospective series and is now being widely utilized as salvage therapy. Given ongoing BCG shortages, as well as the promising efficacy and tolerability of this combination, the objective of this study was to investigate the safety and efficacy of intravesical gemcitabine + docetaxel for high-risk BCG naïve NMIBC in a prospective fashion.
This is a prospective, single-arm, open-label phase 2 trial of patients with high-risk, BCG-naïve NMIBC. Intravesical gemcitabine + docetaxel was given weekly for 6 weeks as induction followed by monthly maintenance therapy for two years among responders. The primary endpoint was 3-months complete response, and key secondary endpoints included adverse events (AEs) and 12-month recurrence-free survival (i.e., 12-month complete response).
Twenty-five patients were enrolled between August 2020 and August 2022, with a median follow-up of 19.6 months. The pre-treatment pathologic stages were HG T1 + CIS (n=7), HG T1 without CIS (n=6), HG Ta (n=9), and CIS alone (n=3).

The 3-months complete response rate was 100% and recurrence-free survival at 12 months was 92%. Two patients with HGT1 disease at trial enrolment had analogous HG T1 recurrences at 9 and 12 months of follow-up.
No patients progressed to T2 disease, underwent radical cystectomy, or had any radiographic evidence of progressive disease.
Grade 1 adverse events were common (23/25 patients) and included hematuria, urinary frequency, urgency, and fatigue. Five patients (20%) experienced a grade 3 adverse event, including hematuria and a urinary tract infection.
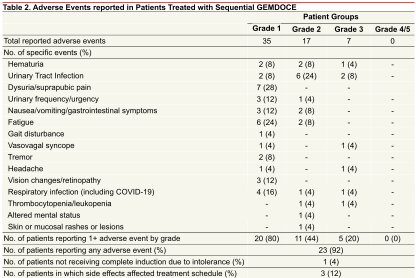
Based on the results of this single-arm phase II trial, Dr. Gabrielson concluded that gemcitabine + docetaxel was well-tolerated with promising efficacy outcomes for patients with high-risk, BCG-naïve NMIBC. These data provide impetus for EA8212 (BRIDGE), a phase 3 randomized controlled trial enrolling patients to either BCG or gemcitabine + docetaxel in high-risk NMIBC patients.
Presented by: Andrew Gabrielson, MD, Resident Physician, Department of Urology, Johns Hopkins Brady Urological Institute, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


