(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a non-invasive bladder cancer moderated poster session. Dr. Vikram Narayan presented an analysis of urinary minimal residual disease detection for predicting recurrent in BCG unresponsive non-muscle invasive bladder cancer and quantifying molecular response to nadofaragene firadenovec.
Urinary minimal residual disease profiling uses next-generation sequencing to identify mutations associated with urothelial carcinoma and can be used to predict recurrence and assess response to therapy. Nadofaragene firadenovec is a novel intravesical therapy recently approved for BCG-unresponsive NMIBC.1 This study sought to evaluate the utility of urinary minimal residual disease to identify molecular response to nadofaragene in patients with high-grade BCG-refractory or relapsed NMIBC.
This was an open-label, multicenter, parallel-arm, phase II study (NCT01687244) of 43 patients with BCG-unresponsive NMIBC who received intravesical nadofaragene:
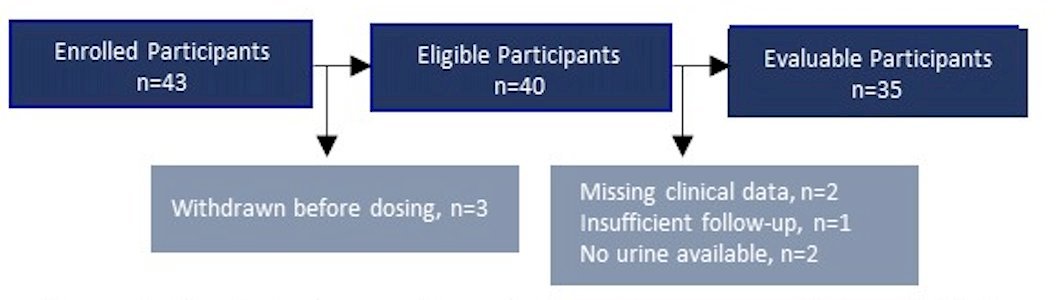
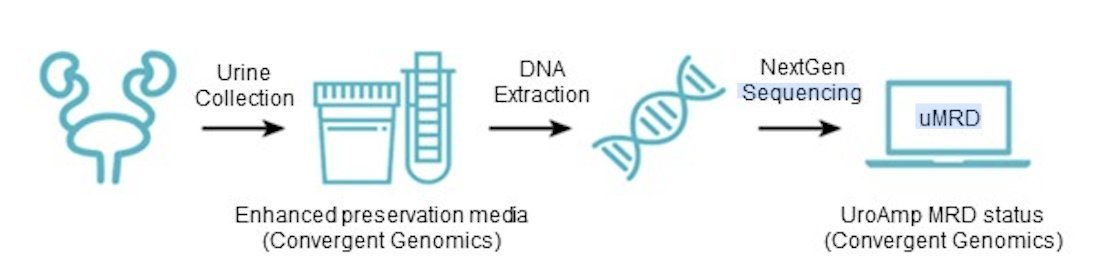
The primary endpoint was 12-months high-grade recurrence-free survival. All patients who received at least one dose were included in the urinary minimal residual disease analysis. Urine samples were collected prior to induction and at 3 months. Urinary minimal residual disease testing was done using the UroAmp MRD assay, which identifies single-nucleotide variants, copy-number variants, insertion-deletions, copy-neutral loss of heterozygosity, microsatellite instability, and aneuploidy:
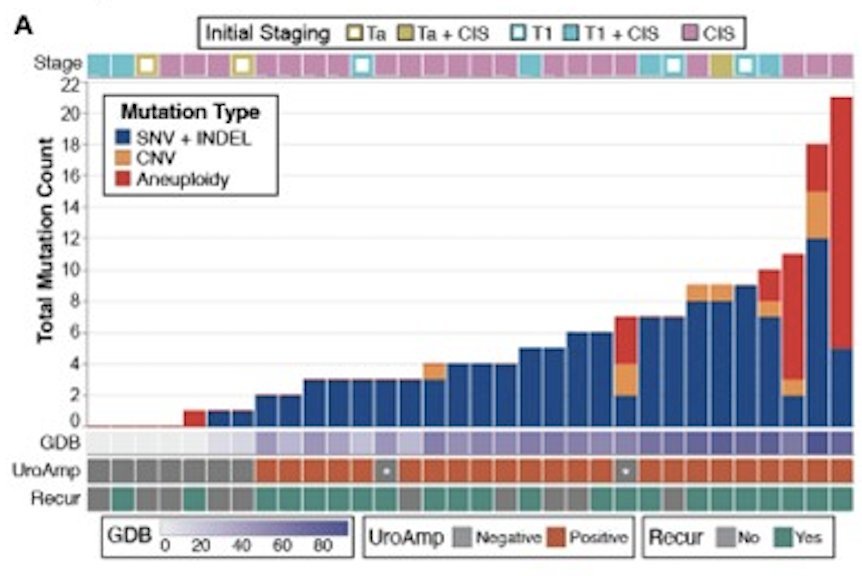
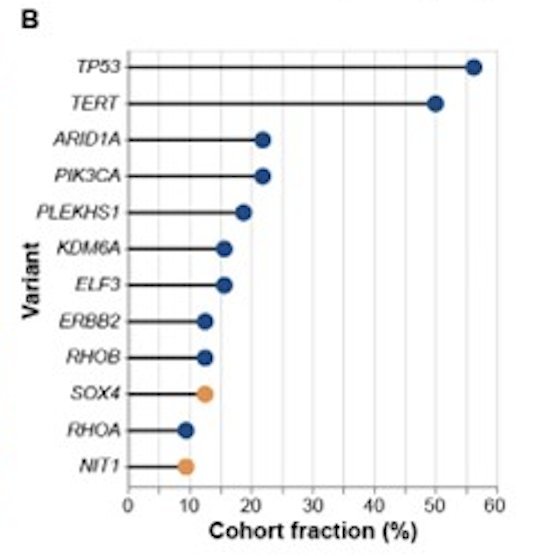
Among evaluable patients (n=35), initial pathological stages were Ta (n=3), T1 (n=9), and Tis (n=23), with concomitant CIS in six patients. Mutation profiles in pre-treatment urine (n=32) were highly varied: TP53, TERT, PIK3CA, ARID1A, PLEKHS1, ELF3, and ERBB2 were among the most prevalently mutated genes:
Most copy-number variants occurred in SOX4 and NIT1:
Urinary minimal residual disease identified patients with high (72%) and low (28%) recurrence risk in both pre-and post-induction collections. At 12 months, post-induction recurrence-free survival rate was 100% for low-risk and 38% for high-risk patients (p = 0.038, log-rank test):
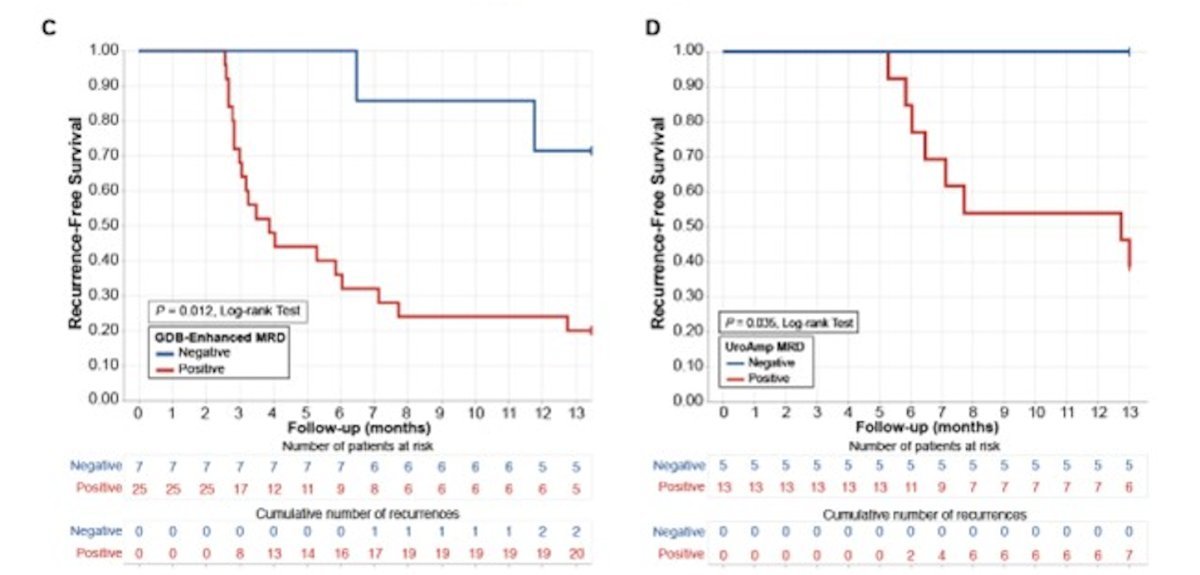
Pre-induction recurrence-free survival was 56% for low-risk and 22% for high-risk (p = 0.097, log-rank test). Using matched pre- and post-induction urine (n=15), quantitative drug response was measured and patients were categorized as:
- Minimal residual disease negative (7%)
- Minimal residual disease complete responder (13%)
- Minimal residual disease partial responder (27%)
- Minimal residual disease stable (20%)
- Minimal residual disease refractory (33%)
Recurrence correlated broadly with response groups: minimal residual disease negative and complete responder groups did not recur on study, while 7 of 12 patients in the other groups recurred:

Dr. Narayan concluded his presentation by discussing urinary minimal residual disease detection predicting recurrence in BCG-unresponsive NIMBC and quantifying molecular response to nadofaragene firadenovec with the following take-home points:
- Urinary minimal residual disease enables quantitative assessment of molecular response to drug treatment
- Urinary minimal residual disease determined pre-treatment disease burden assessment can support stratification of control and intervention arms in future treatment trials
- Post-treatment urinary minimal residual disease status was highly predictive of future recurrence. Those patients who were minimal residual disease negative after nadofaragene induction showed no recurrences
Presented by: Vikram Narayan, MD, Assistant Professor, Department of Urology, Emory University, Winship Cancer Institute, Atlanta, GA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:


