(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a non-invasive bladder cancer moderated poster session. Dr. Benjamin Joffe presented the results of a study evaluating the combination of intravesical docetaxel, gemcitabine, and cisplatin in patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC).
Bacillus Calmette-Guérin (BCG) remains the standard of care approach for high-risk NMIBC. However, approximately 40% of patients who are treated with BCG will experience treatment failure and disease recurrence. While the gold standard approach for patients for whom BCG fails is radical cystectomy, many patients either refuse or are medically unfit for major surgery.
In this study, Dr. Joffe presented the long-term clinical follow-up and sequencing data for select patients with BCG-unresponsive NMIBC who received an off-trial combination of docetaxel, gemcitabine, and cisplatin.
This was a retrospective review of all patients who received gemcitabine + docetaxel + cisplatin from January 2018 to July 2023. This regimen included a 6-week induction of separate-day weekly docetaxel (80 mg), weekly gemcitabine (1000 mg or 2000 mg), and biweekly cisplatin (100 mg). In patients with treatment response, a maintenance regimen of separate-day monthly docetaxel (80 mg) and monthly gemcitabine (1000 mg) was initiated.
Initial follow-up cystoscopy with biopsy was performed 6 weeks after the final instillation; follow-up cystoscopy and cytology were performed every three months thereafter. A complete response was defined as a negative cystoscopy and cytology. Primary outcomes of interest were biopsy-proven recurrence, progression (increase in T stage), metastasis, and/or proceeding to radical cystectomy. Pre- and post-treatment tumors were sent for whole exome sequencing (WES).
A total of 16 patients received gemcitabine + docetaxel + cisplatin for BCG-unresponsive NMIBC. Nearly 45% of patients had HG T1, 56% had concomitant carcinoma in situ, and 13% had variant histology. All patients were BCG unresponsive; 63% had another intravesical agent and 6% had systemic pembrolizumab prior to this combination.
Full induction was completed in 13/16 patients (82%). At first follow-up, 7 patients (44%) had a complete response and proceeded to receive maintenance therapy. Overall, patients were followed for a median of 41 months (IQR: 12–49) after finishing induction. Four of these seven patients (57%) recurred, of whom three underwent gemcitabine + docetaxel + cisplatin reinduction with a complete response re-achieved in one. By the end of the study period, 5/16 patients (31%) had progressed, and 6/16 patients (37%) underwent a radical cystectomy.
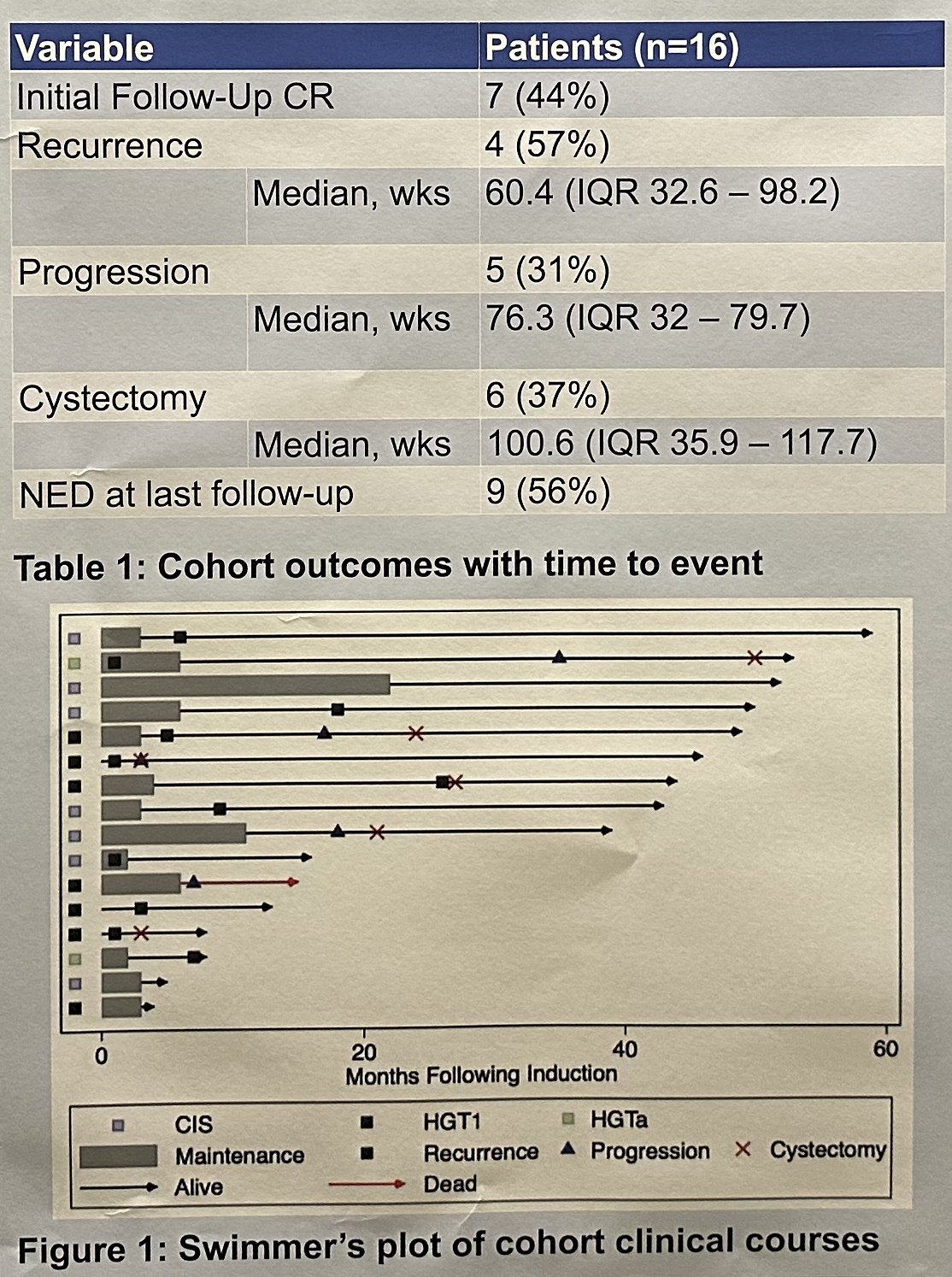
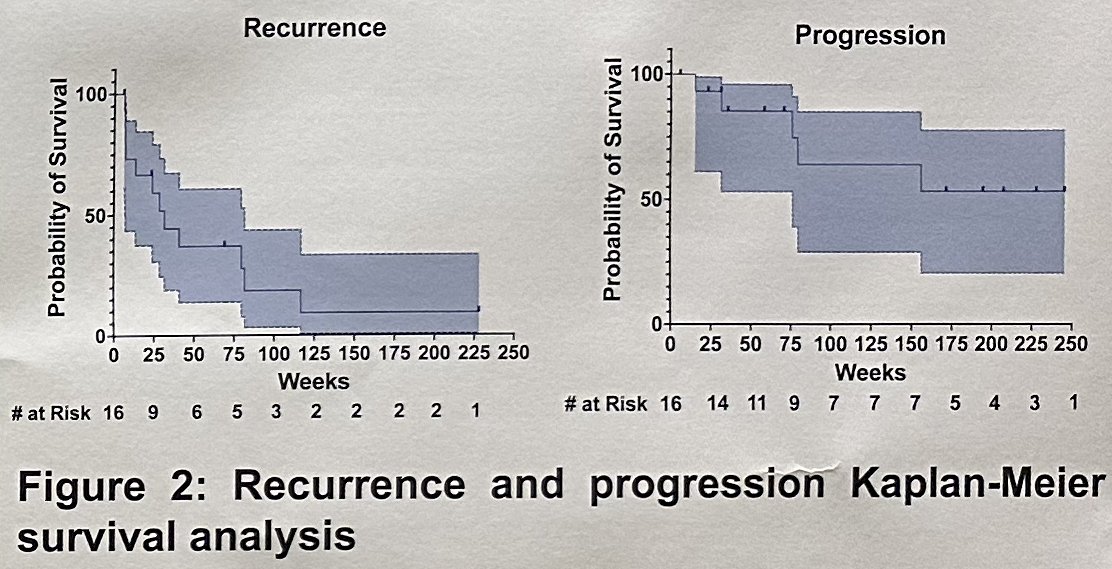
The investigators concluded that in this highly-pretreated cohort of BCG-unresponsive NMIBC, most patients tolerated induction gemcitabine + docetaxel + cisplatin, which resulted in a complete response rate of nearly 45% and approximately two thirds of patients avoided radical cystectomy over the study period.
Presented by: Benjamin Joffe, MD, Resident Physician, Department of Urology, Columbia University Irving Medical Center, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


