(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a non-invasive bladder cancer moderated poster session. Dr. Bernard Bochner presented the results of a phase 1 study of intravesical Fc-optimized anti-CD40 agonist antibody 2141-V11 for non-muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG).
2141-V11 is a novel anti-CD40 antibody developed with enhanced binding to FcγRIIB resulting in effective tumor-specific T-cell responses in vivo. In preclinical bladder cancer models, including BCG-unresponsive disease, intravesical 2141-V11 results in durable anti-tumor immunity without systemic toxicity. Based on these findings, Dr. Bochner and colleagues initiated a first in human phase I/II study of intravesical 2141-V11 for the treatment of BCG-unresponsive NMIBC.
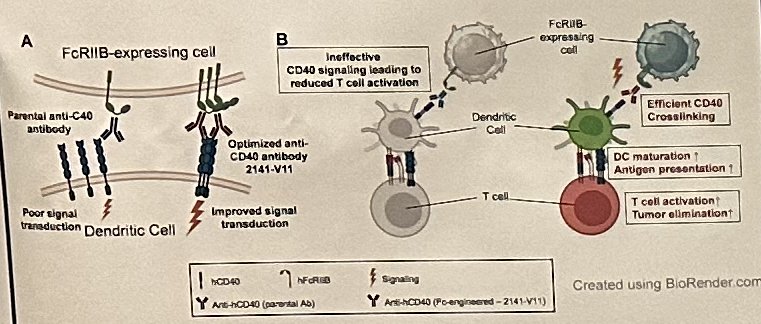
This is an investigator-initiated phase I, open-label, dose-escalation study to evaluate the safety and tolerability of intravesical 2141-V11 in patients with BCG-unresponsive NMIBC who are ineligible for or decline radical cystectomy (NCT05126472; N=25). Following complete transurethral resection, intravesical 2141-V11 was administered once weekly for three doses with re-treatment eligibility at weeks 13 and 25, depending on disease state. Dose escalation follows a modified continual reassessment method (MCRM) design. Primary endpoints were safety and dose tolerability to determine the maximal tolerated dose (MTD) and/or recommended phase II dose. Secondary endpoints include pharmacokinetics and preliminary evaluation of clinical activity. Exploratory aims include investigation of biological markers of drug activity in tissue and urine.
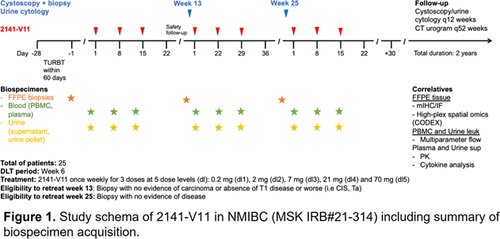
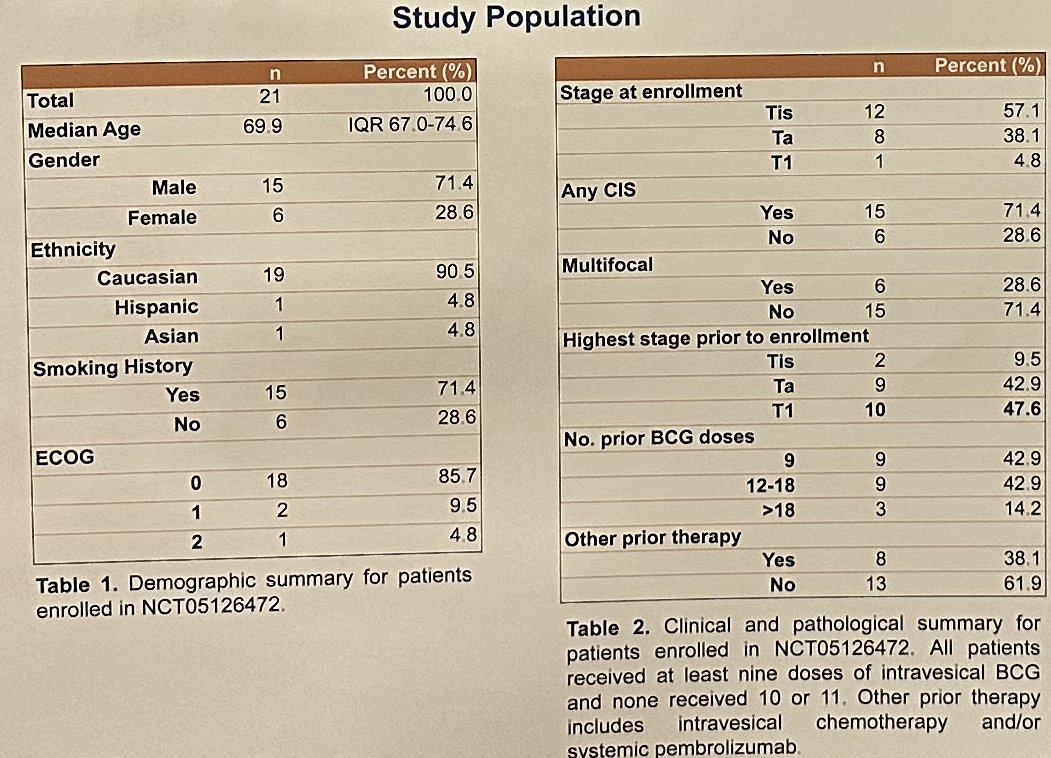
This analysis included 21 patients to date. Patients included had carcinoma in situ (CIS) with or without Ta/T1 (N=15) or Ta/T1 without CIS (N=6). Ten patients had a prior history of T1 disease. Patients received a mean of 12.7 doses of prior BCG.
Intravesical 2141-V11 was well tolerated (no grade ≥3 events) with no dose-limiting toxicities up to the highest tested dose of 70 mg. MTD was not reached.

With regards to efficacy outcomes, at the 3-month timepoint (n=20 evaluable patients), 17/20 did not develop HG T1 disease six (30%) demonstrated a complete response. At the 6-month timepoint (n=16 evaluable patients), 7/16 (44%) had achieved a complete response. A total of 9.20 (45%) demonstrated a complete response at any time point.

Post-treatment urine studies reveal increased neutrophils/neutrophil-related cytokines, particularly in responders. Single-cell spatial phenotyping is ongoing.
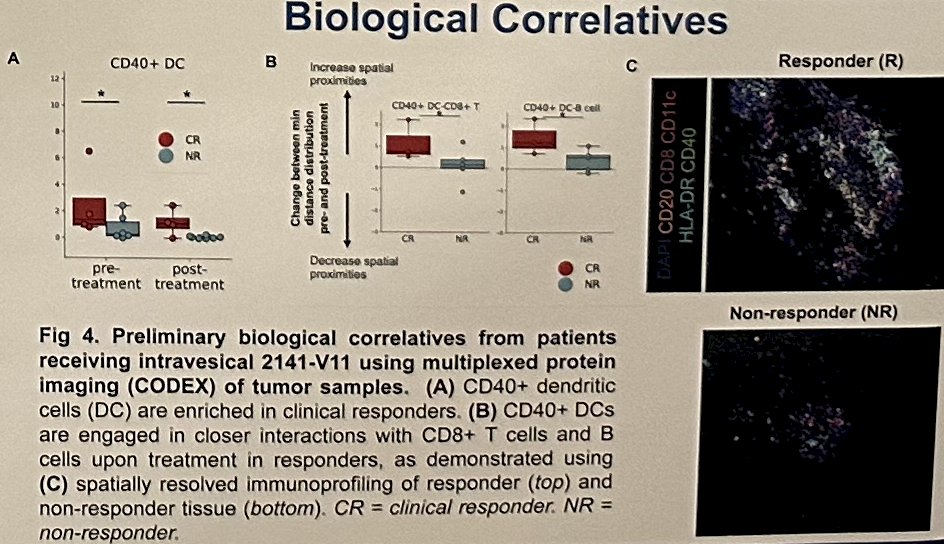
Dr. Bochner concluded that intravesical 2141-V11 is well-tolerated with no dose-limiting toxicities observed up to 70 mg. MTD was not reached. Despite nearly half the population having a history of T1 disease prior to enrollment, 44% demonstrated a complete response at 6 months with a complete response rate of 45% at any time point. Correlative studies are ongoing to further investigate on-target activity in post-treatment specimens.
Presented by: Bernard Bochner, MD, FACS, Professor, Sir Murray Brennan Endowed Chair in Surgery, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


