(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX 2024 was host to a non-invasive bladder cancer podium session. Dr. William Huang presented a post hoc analysis of the ATLAS trial evaluating the response to primary chemoablation with UGN-102 in patients with new or recurrent low-grade, intermediate-risk non-muscle invasive bladder cancer (NMIBC).
Patients with low-grade, intermediate-risk NMIBC are at low risk of disease progression; however, disease recurrence is common and occurs in >50% of patients requiring repeated TURBT. This procedure commonly requires general anesthesia, utilizes healthcare resources, and may increase patient morbidity and mortality. Furthermore, while clinical guidelines recommend adjuvant therapy in the post-TURBT setting to reduce disease recurrence and, potentially, disease progression, compliance with such practices remains low. As such, there is a clear need for primary non-surgical chemoablative treatment options for such patients with low-grade, intermediate-risk NMIBC.
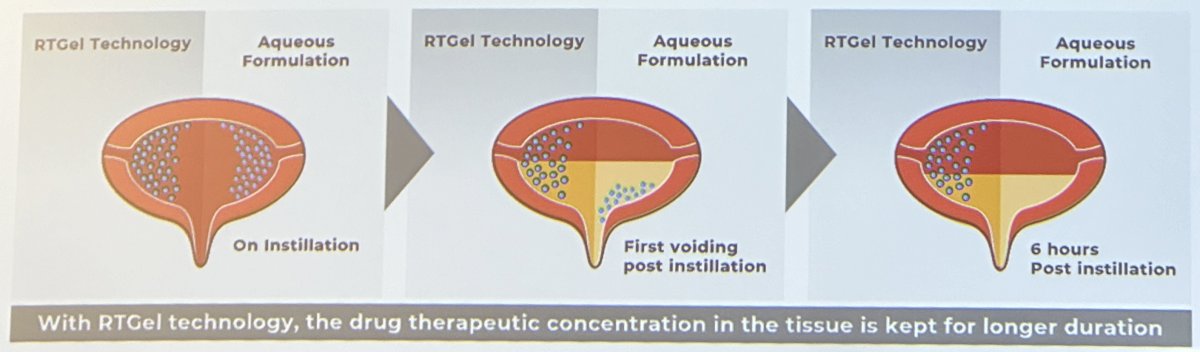
UGN-102 is an investigational drug consisting of mitomycin-C combined with a proprietary reverse thermal hydrogel used to reconstitute mitomycin prior to instillation. The reverse thermal properties of UGN-102 allow for the local administration of mitomycin as a liquid in a cooled state, with subsequent conversion to a semi-solid gel depot at body temperature post-instillation. The gel slowly disintegrates and is subsequently eliminated by voiding, allowing for the sustained release of mitomycin over four to six hours. The prolonged exposure of tumor cells to mitomycin is hypothesized to improve chemoablation compared with aqueous preparations of the drug. This drug is administered intravesically once weekly for 6 weeks.
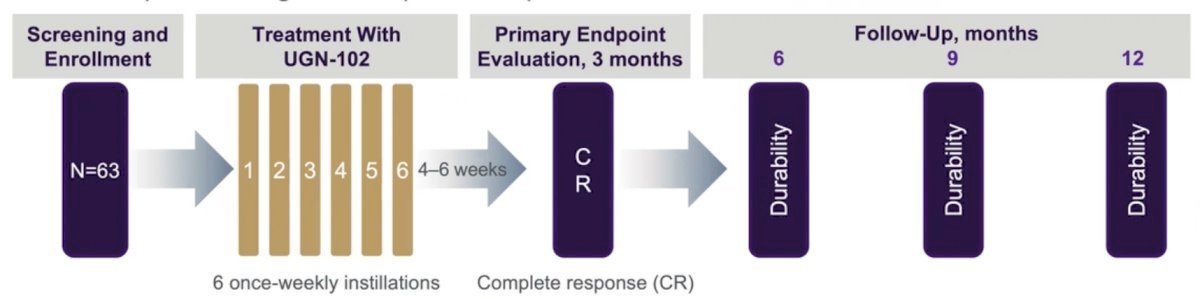
In the phase 2b, open-label, single-arm, multicenter OPTIMA II trial of UGN-102 primary chemoablation in patients with biopsy-proven low-grade intermediate-risk NMIBC, UGN-102 was associated with a 65% complete response rate at 3 months, with 61% of patients remaining disease-free 12 months following treatment initiation.1 Patients who completed OPTIMA II and participated in a rollover study had a 24.4-month median duration of response.1
ATLAS is a prospective, phase 3, randomized, open-label trial that randomized patients with newly diagnosed or recurrent low-grade, intermediate risk NMIBC in a 1:1 fashion to either UGN-102 +/- TURBT or TURBT alone (no adjuvant therapy). Eligible patients were those diagnosed via cold cup biopsy, with visible tumors left in situ, and had a voiding cytology negative for high-grade disease. Intermediate-risk disease was defined as having one or two of the following three risk factors:
- Presence of multiple tumors
- Solitary tumor >3 cm
- Recurrence (≥1 occurrence of low-grade NMIBC within one year of the qualifying diagnosis)
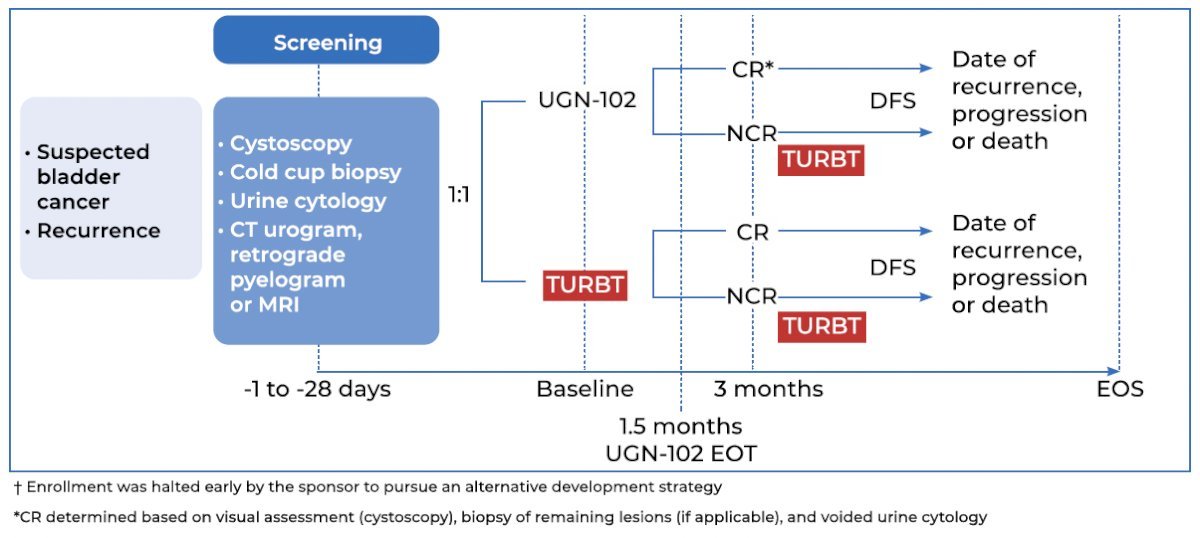
Patients in the UGN-102 arm of ATLAS received six weekly intravesical instillations. Patients in this arm with a complete response at 3 months received no further treatment, whereas those with residual low-grade disease in either treatment arm underwent a TURBT for any remaining lesions.
Of note, following the enrollment of 282 of the planned 632 study participants, further study enrollment was suspended early by the sponsor to pursue an alternative development strategy for UGN-102 for the treatment of bladder cancer. Patients who had consented at the time of trial termination were permitted to continue, but follow-up was discontinued once the last patient had been followed for 15 months after treatment initiation.
Analysis of the overall patient population was published in The Journal of Urology in 2023. At the first disease assessment 3 months post-treatment initiation, a complete response was observed in:
- UGN-102 +/- TURBT arm: 65% (95% CI: 56 – 73%)
- TURBT alone arm: 64% (95% CI: 55 – 72%)
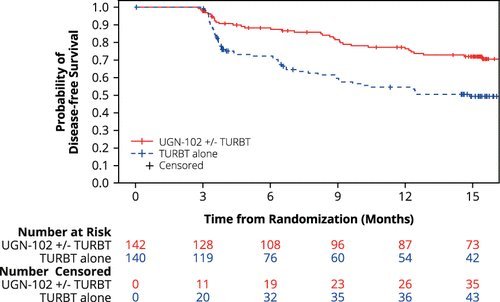
Disease-free survival 15 months after randomization was estimated to be 72% for patients in the UGN-102 +/- TURBT arm and 50% for patients in the TURBT monotherapy arm, with a hazard ratio of 0.45:
Among patients achieving a complete response at the 3-months assessment, the estimated 12-months maintained response was 80% following induction treatment with UGN-102 and 68% following primary TURBT, with a hazard ratio of 0.46:
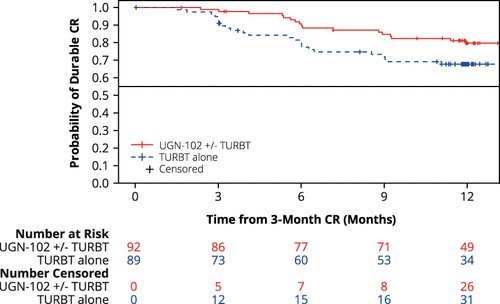
In this report, Dr. Huang presented the results of a pre-specified subgroup analysis, whereby the durations of response and disease-free survivals were compared between newly diagnosed and recurrent low-grade intermediate-risk NMIBC patients treated with UGN-102 +/- TURBT.
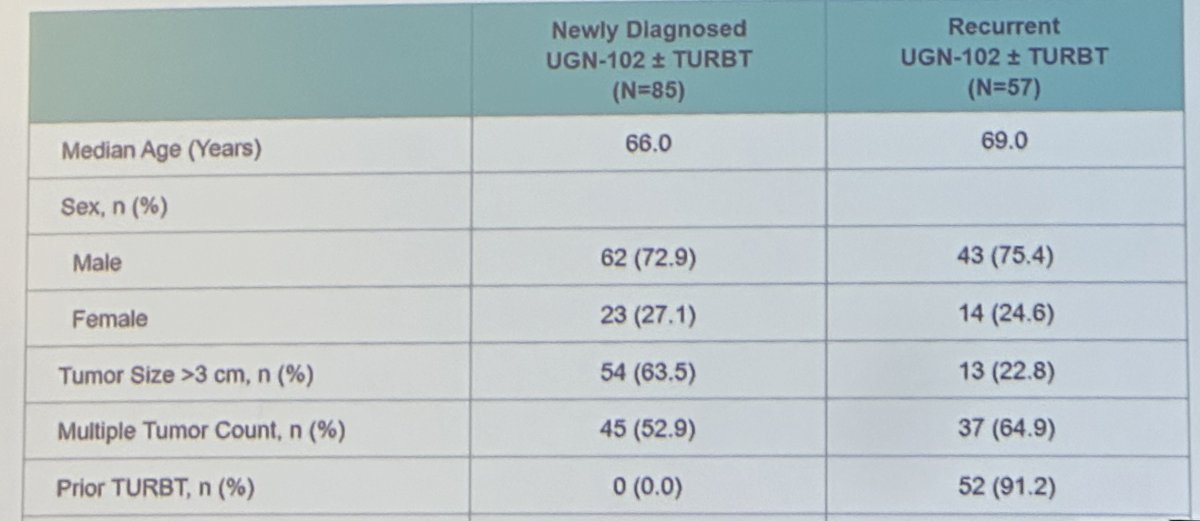
Demographic and baseline characteristics were well-balanced between the two subgroups (newly diagnosed versus recurrent):
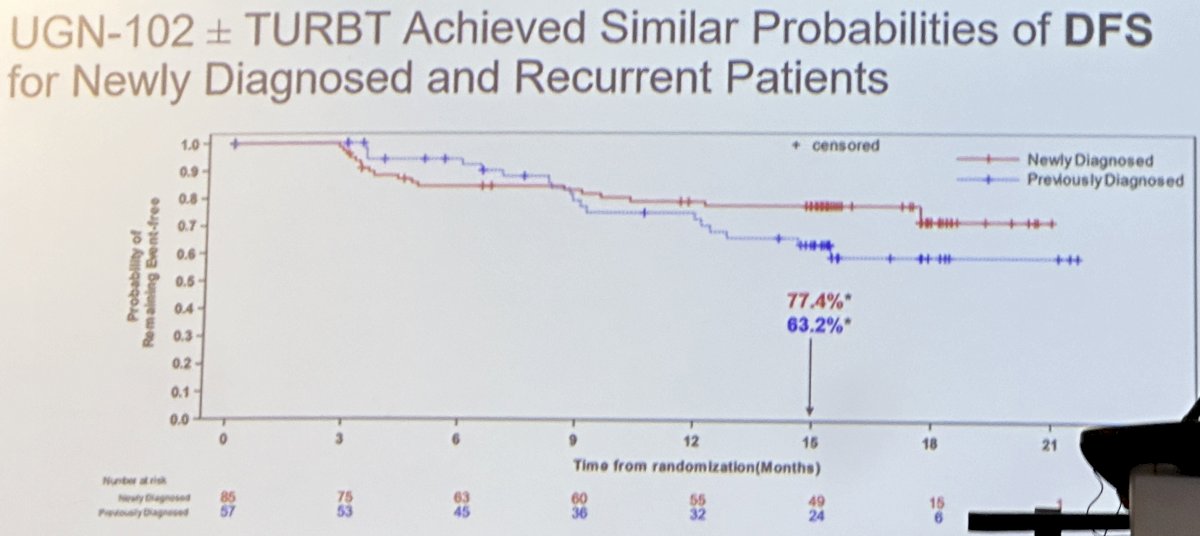
As demonstrated in the Kaplan-Meier curve below, UGN-102 treatment led to similar disease-free survival rates among patients with newly diagnosed or recurrent low-grade intermediate-risk NMIBC.
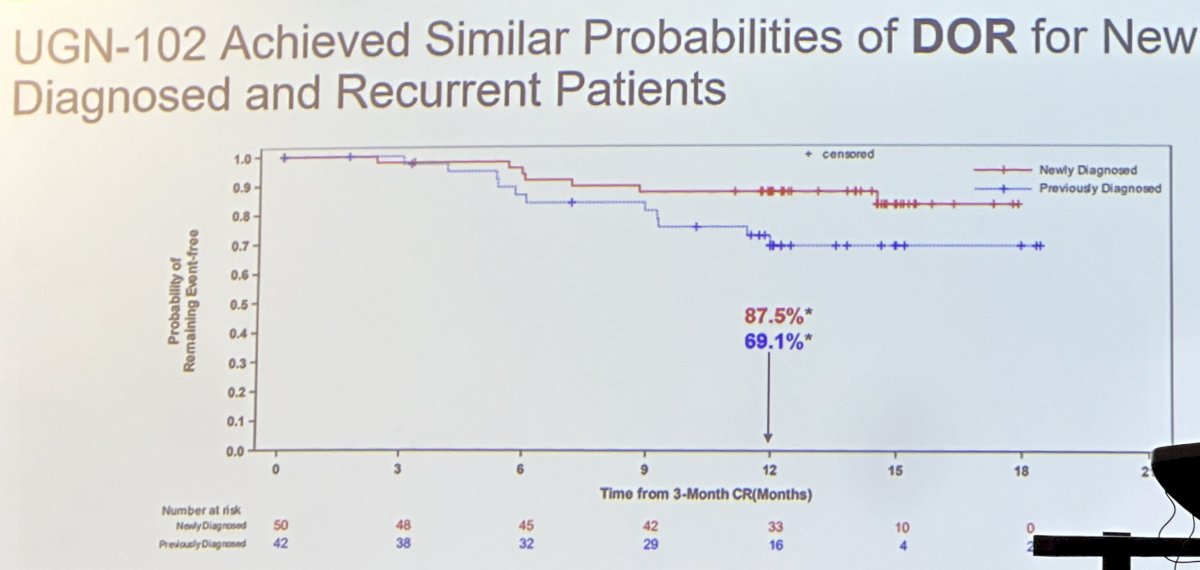
Among patients who achieved a response, the durations of response were similar for newly diagnosed and recurrent patients treated with UGN-102 +/- TURBT, albeit slightly favoring the newly diagnosed patients.
Adverse event rates were similar in both groups, as summarized below:
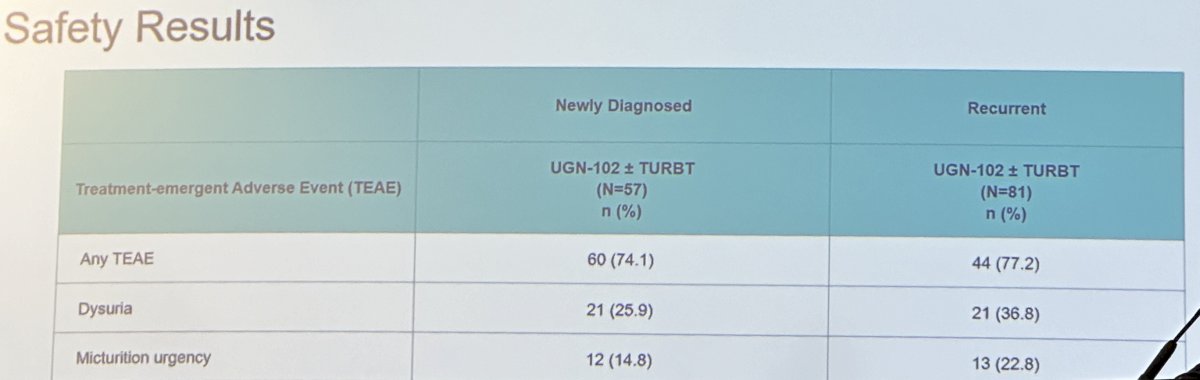
Dr. Huang concluded that:
- This analysis demonstrates that UGN-102 +/- TURBT results in meaningful and similar durations of response and disease-free survivals in patients with newly diagnosed and recurrent low-grade, intermediate-risk NMIBC
- UGN-102 is being assessed in the ongoing phase 3, single arm ENVISION study (NCT05243550)
- UroGen anticipates completing the submission of the rolling NDA for UGN-102 in 2024 with potential FDA decision as early as Q1 2025
Presented by: William Huang, MD, Professor of Urology and Radiology, NYU Langone Health, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Chevli KK, Shore ND, Trainer A, et al. Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial. J Urol 2022; 207(1):61-9.
- Prasad SM, Huang WC, Shore ND, et al. Treatment of Low-grade Intermediate-risk Nonmuscle-invasive Bladder Cancer With UGN-102 ± Transurethral Resection of Bladder Tumor Compared to Transurethral Resection of Bladder Tumor Monotherapy: A Randomized, Controlled, Phase 3 Trial (ATLAS). J Urol 2023;210(4): 619-23.


