(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the muscle-invasive bladder cancer podium session. Dr. Guru Sonpavde presented the results of CheckMate 901, a phase 3 trial evaluating the combination of nivolumab plus gemcitabine/cisplatin (GC) versus gemcitabine/cisplatin alone for patients with previously untreated unresectable or metastatic urothelial carcinoma (mUC), in his presentation he characterized patients who responded to treatment.
Dr. Sonpavde opened his presentation by highlighting that cisplatin-based chemotherapy has been the first-line standard of care option for eligible patients with unresectable or mUC for decades, generating robust response rates (~40%) but not durable responses, with a median overall survival (OS) of ~15 months. Avelumab has shown a survival benefit as maintenance therapy, but only for the subset of patients who did not experience disease progression with first-line platinum-based chemotherapy.1 The phase 3 CheckMate 901 trial evaluated upfront combination therapy with nivolumab plus gemcitabine/cisplatin (NIVO+GC) followed by nivolumab (NIVO) monotherapy versus GC alone in cisplatin-eligible patients.2 Dr. Sonpavde informed the audience that in March 2024, the US Food and Drug Administration approved NIVO+GC for first-line treatment of patients with unresectable or mUC based on results from this trial.
In this trial, 608 patients underwent 1:1 randomization, stratified by tumor PD-L1 expression and presence/absence of liver metastases, to either:
- Nivolumab 360 mg on D1 + gemcitabine (1,000 mg/m2) on D1/8 + cisplatin (70 mg/m2) on D1 in 3-week cycles, up to a total of 6 cycles. Followed by:
- Nivolumab maintenance at 480 mg every 4 weeks was continued as maintenance therapy in responders until progression, unacceptable toxicity, withdrawal, or up to 24 months.
- Gemcitabine + cisplatin at the same doses/schedule/cycles
The co-primary endpoints were OS and progression-free survival (PFS), per blinded, independent, central review (BICR).
In all responders (n=306), baseline patient characteristics were well-balanced between the NIVO+GC arm (175 patients) and the GC alone arm (131 patients). The median patient age was 65 years in the NIVO+GC arm compared to 64 in the GC alone arm. The primary tumor site was the bladder in 75% of patients in the NIVO+GC arm and 71% in the GC arm. Additionally, 38% of patients had high tumor PD-L1 expression (defined as ≥1%) in the NIVO+GC arm, while 33% had it in the GC arm. Live metastases were observed in 15% of patients in both groups.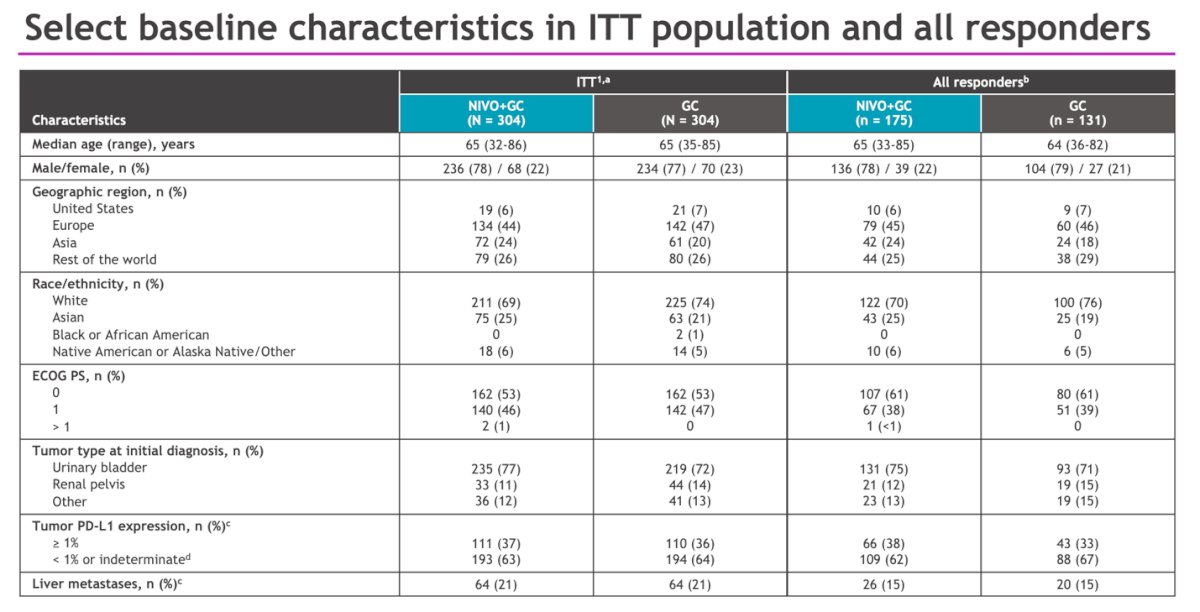
The median duration of study therapy was 7.4 months in the NIVO+GC arm, compared to 3.7 months in the GC arm. In the NIVO+GC arm, 74% of patients completed all 6 cycles of therapy, whereas in the control arm, only 55% of patients did so. A total of 244 patients (80%) randomized to NIVO + GC received maintenance nivolumab, with 8% completing therapy and 9% still on therapy at the time of data cut-off. Among responders, the median duration of study therapy was 11.0 (IQR 0.7-47.9) months in the NIVO+GC arm and 3.9 (IQR 1.9-14.3) months in the GC arm.
Dr. Sonpavde then demonstrated that the study met its primary endpoint of OS, showing a median improvement of nearly 3 months (21.7 versus 18.9 months; HR: 0.78, 95% CI: 0.63 – 0.96, p=0.017) in the intention-to-treat (ITT) population. Additionally, the other co-primary endpoint of PFS per BICR was also significant, with a median PFS improvement from 7.6 to 7.9 months (HR: 0.72, 95% CI: 0.59 – 0.88, p=0.0012).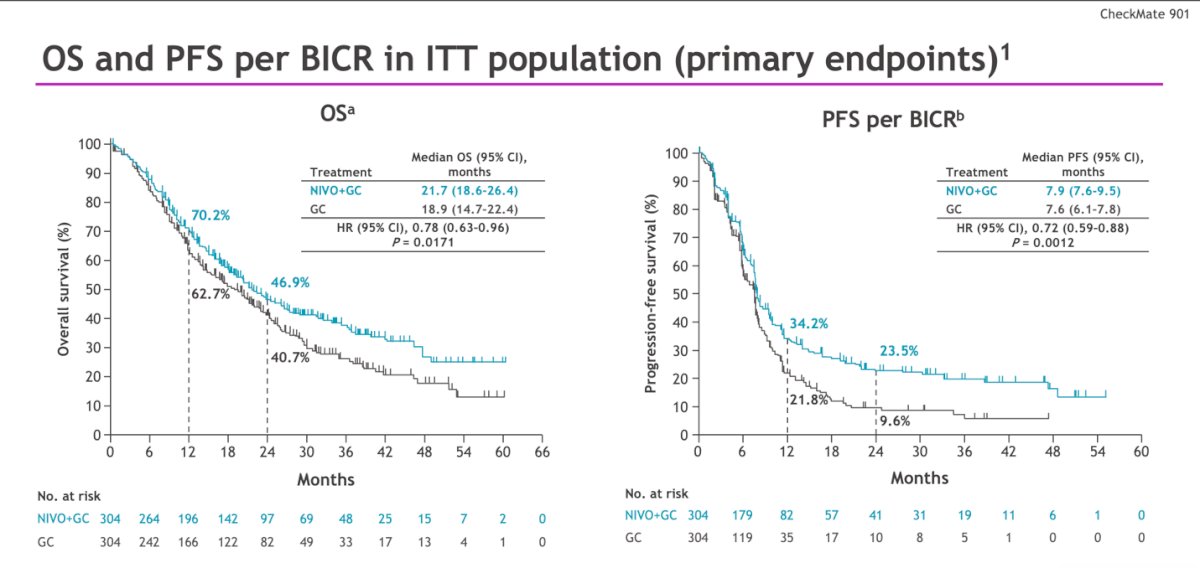
The objective response rate (ORR) was higher in the experimental arm (57.6% versus 43%), with a greater proportion of patients achieving a complete response with NIVO + GC (21.7% versus 11.8%). However, there was no difference in the median time to response (2.1 vs 2.1 months) between treatment arms in all responders.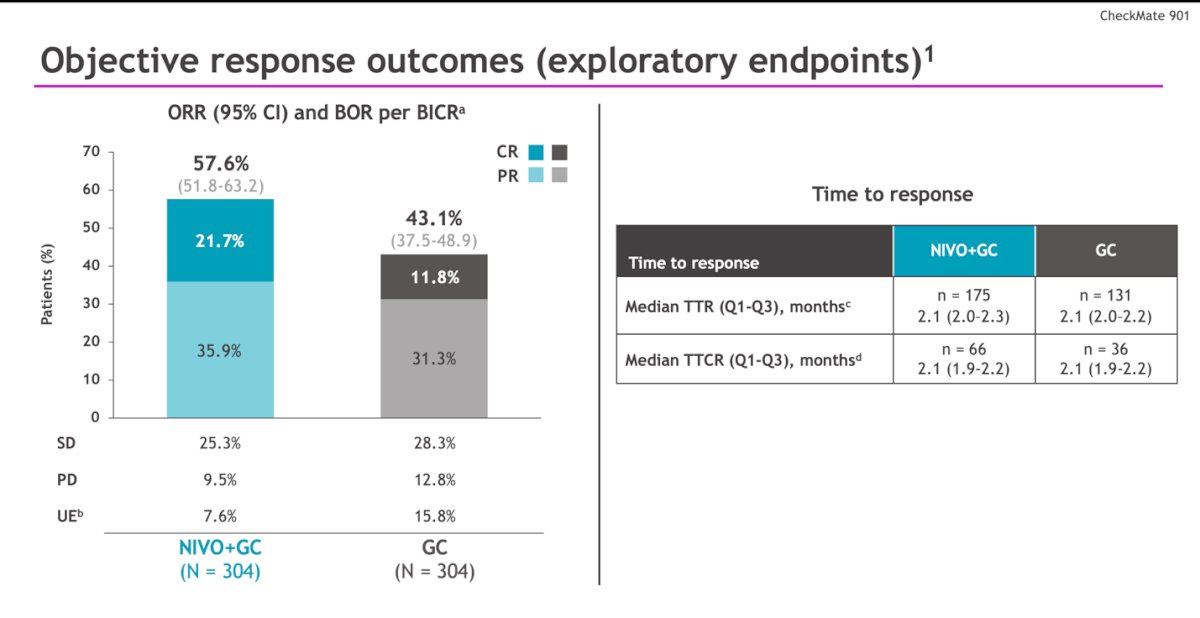
The median duration of response was 9.5 and 7.3 months, and the duration of complete response was 37.1 vs. 13.2 months in the NIVO + GC vs. GC arms, respectively.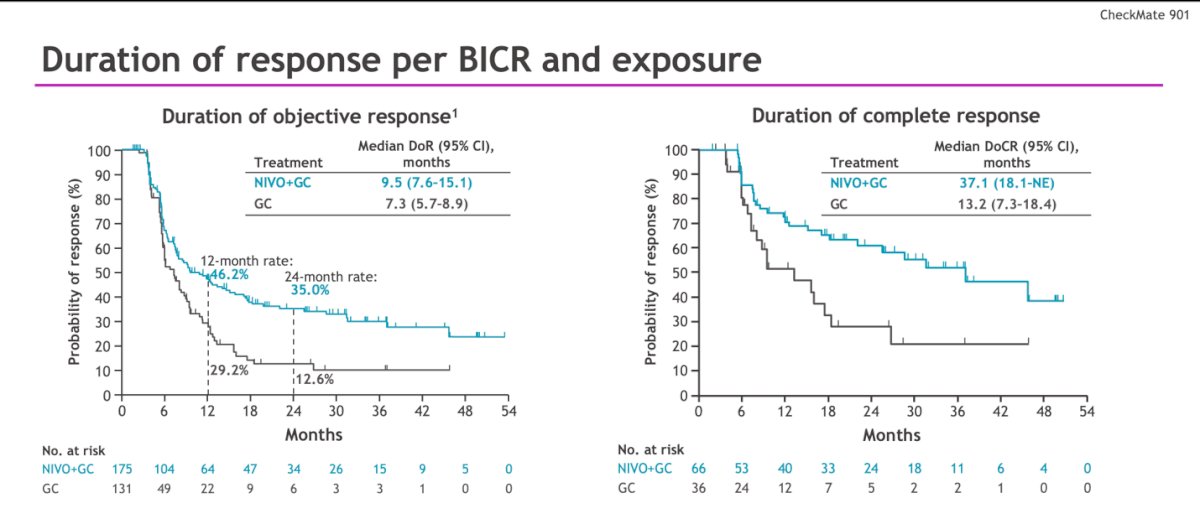
In the responders on the NIVO +GC arm, there was a ≥50%, ≥75%, and ≥90% reduction in sum diameter of target lesions per BICR in 84%, 48%, and 28% of the patients respectively.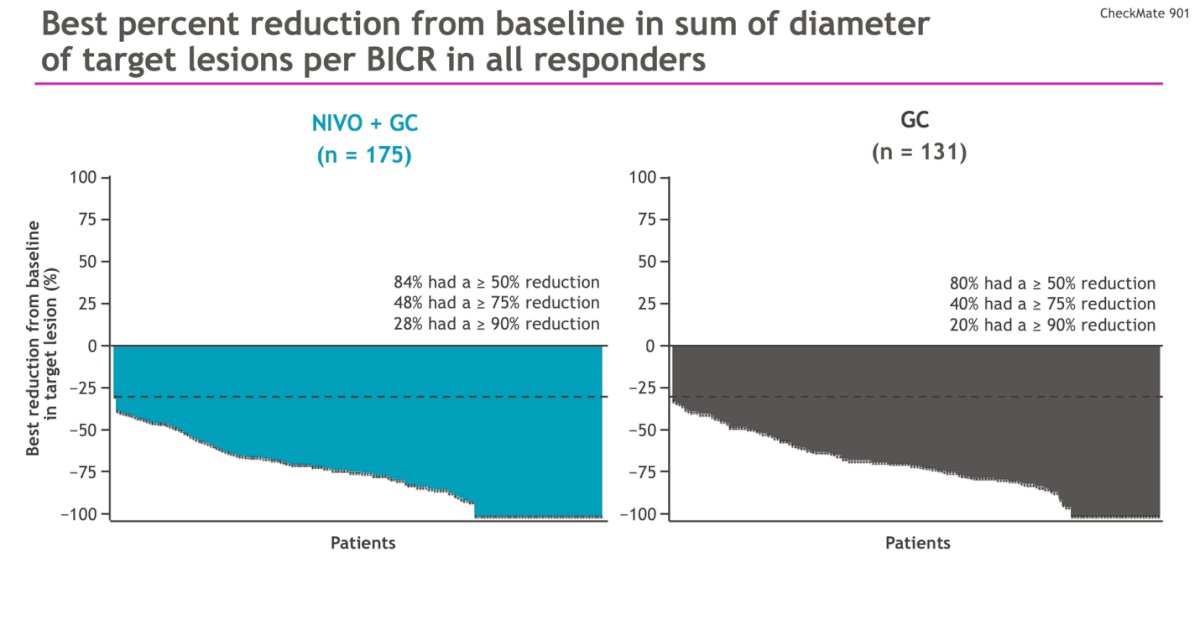
The proportions of patients receiving subsequent ICI in all responders was different in the NIVO+GC arm (9%) compared to the GC alone arm (52%).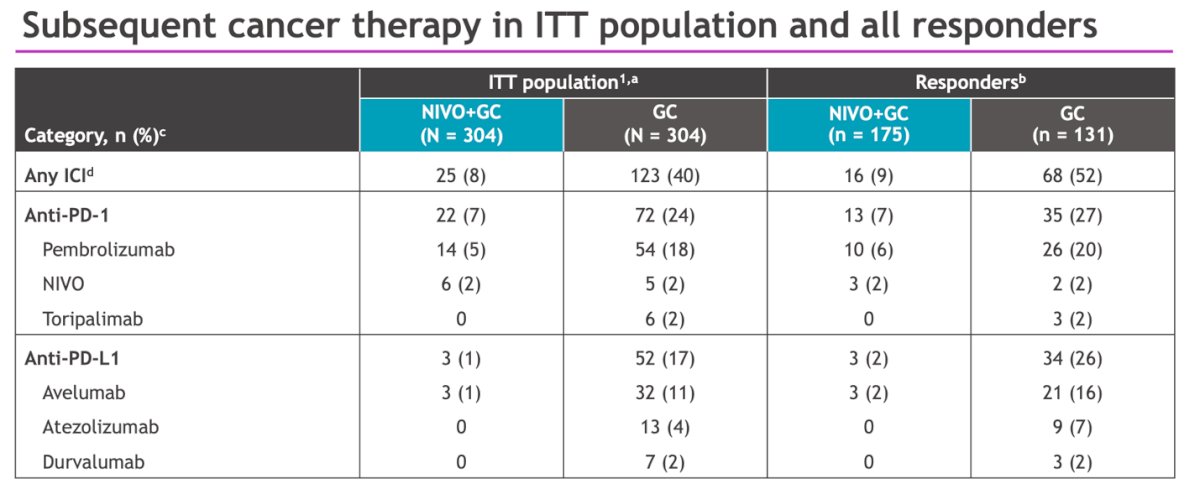
Grade ≥3 treatment-related adverse events (TRAEs) occurred in 61.8% and 51.7% of patients in the experimental and control arms, respectively. TRAEs leading to treatment discontinuation occurred in 11% and 8%, respectively. The most common TRAEs in all responders with NIVO +GC were anemia (50%), nausea (57%), and neutropenia (41%), this TRAEs were slightly more incident than in the ITT population.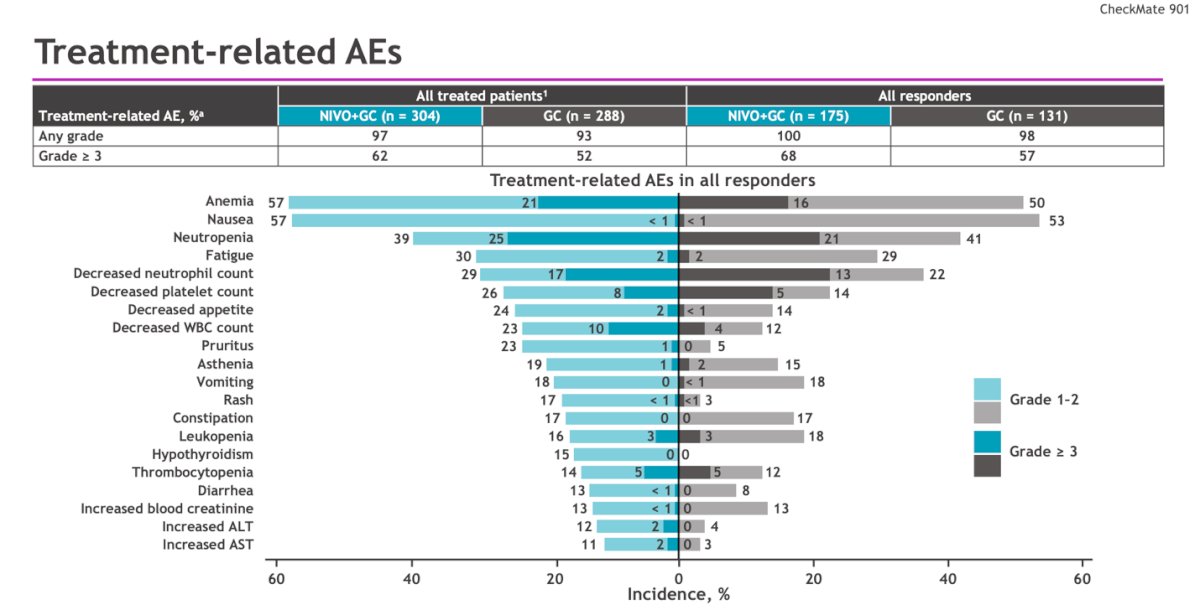
Dr. Sonpavde concluded his talk by saying:
- NIVO+GC showed significant and clinically meaningful benefits in OS (HR, 0.78), PFS (HR, 0.72) compared to GC alone as the first-line treatment for unresectable or mUC.
- The ORR was improved with NIVO+GC versus GC (57.6% vs 43.1%) and the CR rate was almost doubled (21.7% vs 11.8%)
- The duration of CR was almost 3 times longer (median 37.1 vs 13.2 months) with NIVO+GC
- Safety in the responder’s was consistent with previous data in the ITT population, with no new safety signals identified.
Presented by: Guru P. Sonpavde, MD, Medical Director of Genitourinary Oncology, AdventHealth Cancer Institute, Orlando, FL
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
References:- Powles, Thomas, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. New England Journal of Medicine 383.13 (2020): 1218-1230.
- van der Heijden, Michiel S., et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. New England Journal of Medicine 389.19 (2023): 1778-1789.


