(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Bladder Cancer Invasive Podium Session. Dr. Rikiya Taoka presented the results of a Prospective Multicenter Cohort Study assessing peripheral neuropathy and nerve electrophysiological changes in patients with advanced urothelial carcinoma treated with Enfortumab Vedotin.
Dr. Taoka began his presentation by discussing Enfortumab vedotin (EV), a novel antibody-drug conjugate directed against nectin-4, this antibody-drug complex is composed of a fully human monoclonal antibody specific for nectin-4 and monomethyl auristatin E (an agent that disrupts microtubule formation). EV is used as a systematic therapy in the treatment of urothelial cancer. The aim of their study was to examine the impact of EV-induced peripheral neuropathy on its effectiveness and determine if early electrophysiological alterations are linked to the onset of peripheral neuropathy.
For this analysis the investigators used their prospective multicenter cohort study, they enrolled 34 patients with prior platinum-based chemotherapy exposure and programmed cell death protein 1/ligand 1 inhibitor (PD1/PDL1) resistant advanced urothelial carcinoma who received EV. The outcomes of interest of the study were the best overall response, progression-free survival, overall survival, and safety of EV. Nerve conduction studies were also performed for 11 patients. Patient characteristics for the overall population, at baseline, are shown in Table 1.
Patient and clinical characteristics were also broken down into groups based on whether patients developed peripheral neuropathy or not, as shown in Table 3 below. Interestingly, patients with ECOG PS >2 did not develop peripheral neuropathy in this study.
Dr. Taoka presented the oncological outcomes of EV in these 34 patients during a median follow-up of 8.6 months.:
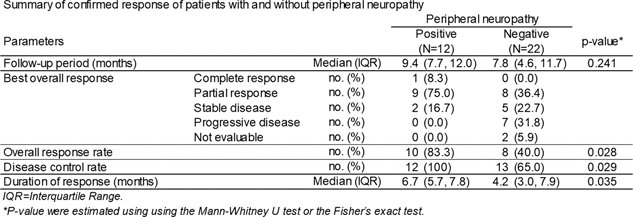
- The confirmed overall response rate was 53% and disease control rate was 73.5%
- The median overall progression-free survival was 6.9 months
- The median overall survival was 13.5 months
Patients who achieved disease control experienced notably prolonged treatment continuation and overall survival compared to those with uncontrolled disease (p=0.035).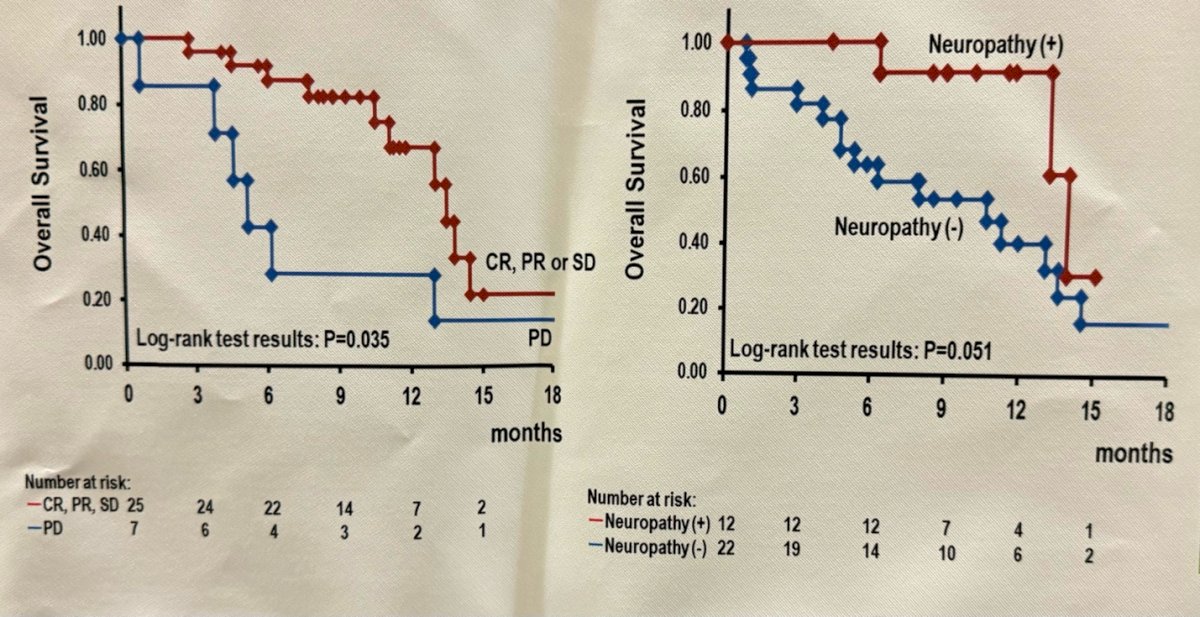
Dr. Taoka then went on to present that peripheral neuropathy occurred in 12.5% of patients, and the overall response and disease control rates for these patients were 83.3 and 100%, respectively, which were higher than those in patients who did not experience peripheral neuropathy (p=0.051).
Nerve conduction studies exploring electrophysiological changes indicated that EV reduced nerve conduction velocity more markedly in sensory nerves than in motor nerves (p=0.070), and more commonly in the lower extremities than in the upper extremities (p=0.027). Interestingly, the sural nerve was the most affected nerve in patients who developed peripheral neuropathy (p=0.011). A summary of nerve conduction studies is shown in the table below.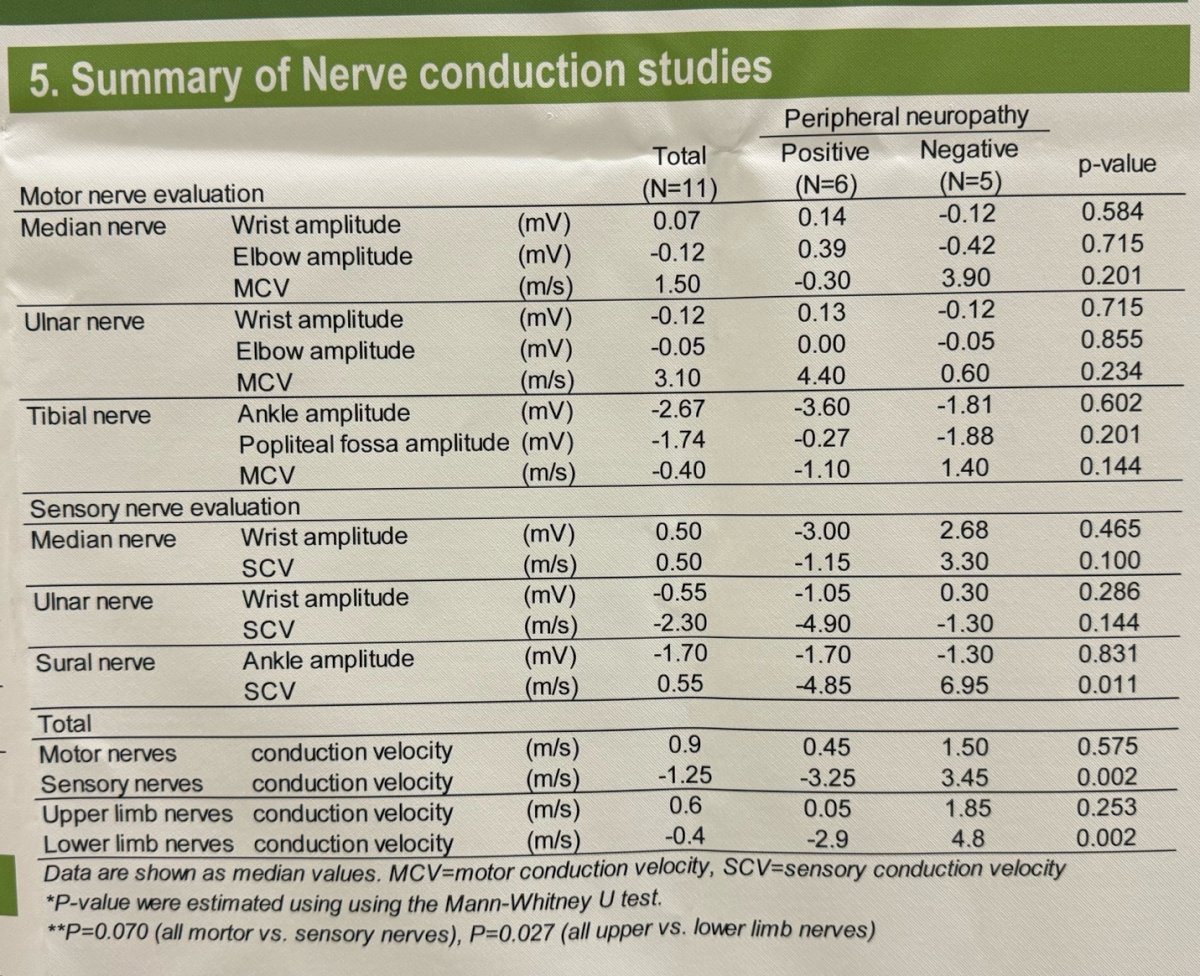
Dr. Taoka made these final remarks about his talk:
- Enfortumab vedotin-related peripheral neuropathy was associated with disease response.
- EV resulted in a more pronounced reduction in nerve conduction velocity, particularly affecting sensory nerves and the lower extremities.
- Their results suggest the importance of focusing on the sural nerve neuropathy to maximize efficacy and improve the safety of EV treatment in advanced urothelial carcinoma.
Presented by: Rikiya Taoka, MD, PhD, Assistant Professor, Department of Urology, Kagawa University, Takamatsu, Japan
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024


