(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on bladder cancer trials in progress, and a presentation by Dr. Roger Li discussing MoonRISe-1, a phase 3 study of TAR-210 versus intravesical chemotherapy in patients with intermediate-risk non–muscle-invasive bladder cancer with susceptible FGFR alterations.
Despite available treatment options for patients with intermediate-risk non-muscle invasive bladder cancer, recurrence rates remain high, underscoring the need for effective therapies. FGFR alterations are prevalent in ~50% to 80% of low-grade non-muscle invasive bladder cancer and may function as oncogenic drivers. Erdafitinib is a selective pan-FGFR tyrosine kinase inhibitor that is approved in the United States to treat adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 alterations following progression on or after at least one prior systemic treatment. Oral erdafitinib has demonstrated clinical efficacy in high-risk and intermediate-risk non-muscle invasive bladder cancer population but is limited by challenging systemic toxicities. TAR-210 is designed for sustained intravesical delivery of erdafitinib over 90 days while limiting systemic toxicities: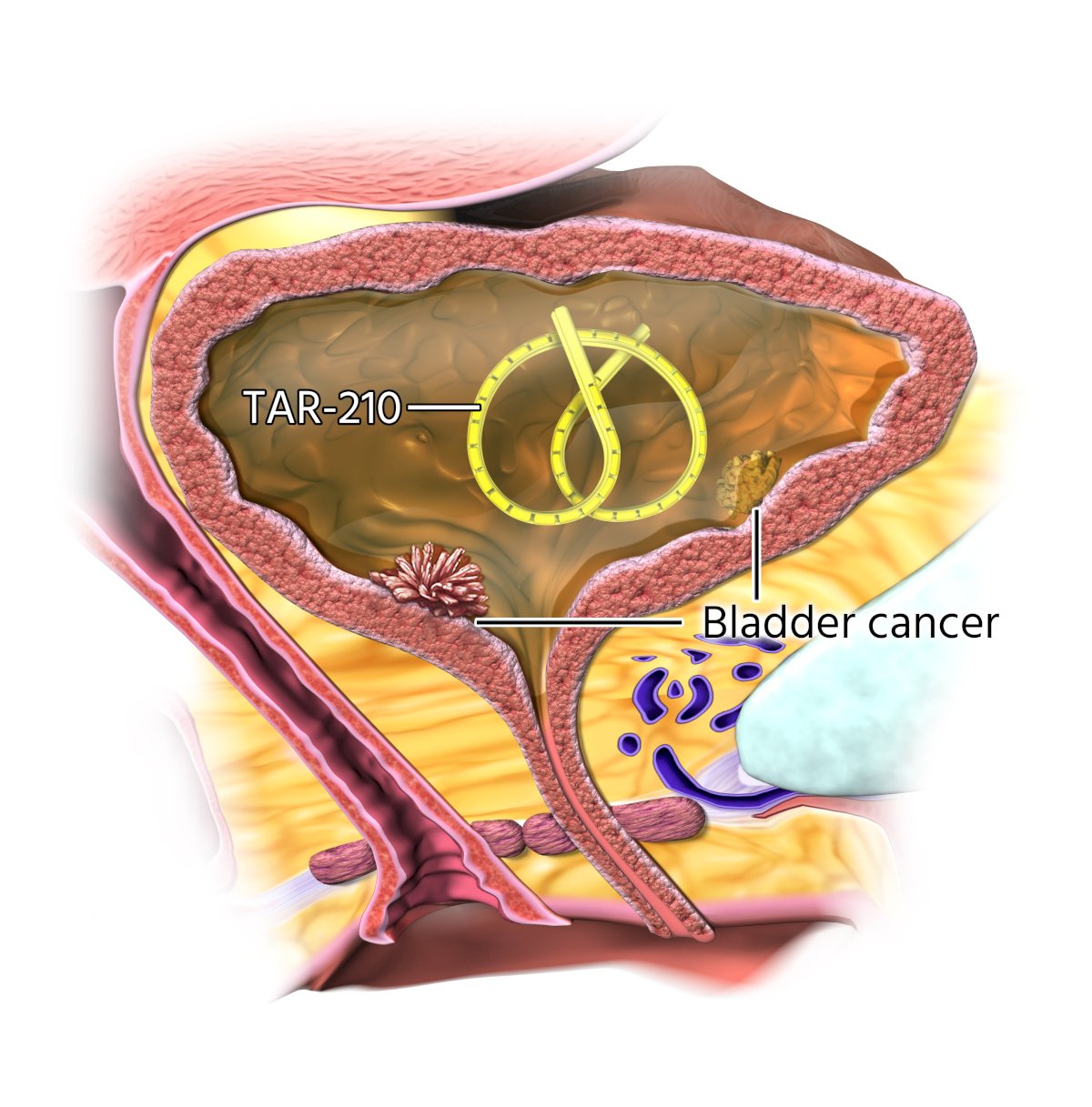
TAR-210 is inserted into the bladder through a dedicated urinary placement catheter and removed via cystoscopy. In a first-in-human study, TAR-210 was well tolerated and has shown promising clinical activity in FGFR-altered high-risk nonmuscle invasive bladder cancer (radiographic progression-free survival rate: 82%) and intermediate risk nonmuscle invasive bladder cancer (complete response rate: 87%).1
Patients in MoonRISe-1 had intermediate risk non-muscle invasive bladder cancer, as well as FGFR2/3 alterations by central or local tissue or urine testing. Patients (n = 540) were then randomized 1:1 to TAR-210 versus investigator’s choice of intravesical chemotherapy (mitomycin C or gemcitabine). The study design assumes an 11% absolute improvement in the 2-year disease-free rate of the TAR-210 group over the chemotherapy group (ie, 80% vs. 69%, a hazard ratio of 0.60) under the exponential distribution assumption. The primary endpoint is disease-free survival and the trial design for MoonRISe-1 is as follows:
Of note, all visible papillary disease must be fully resectioned prior to randomization. Assessments of recurrence or progression include urine cytology, cystoscopy, for cause TURBT or biopsy of bladder lesions, ultrasound, and urography. The follow-up phase for patients meeting the primary endpoint is up to ~5 years.
The MoonRISe-1 study opened for enrollment on April 10, 2024, with enrollment planned at 200 sites in 20 countries across four continents: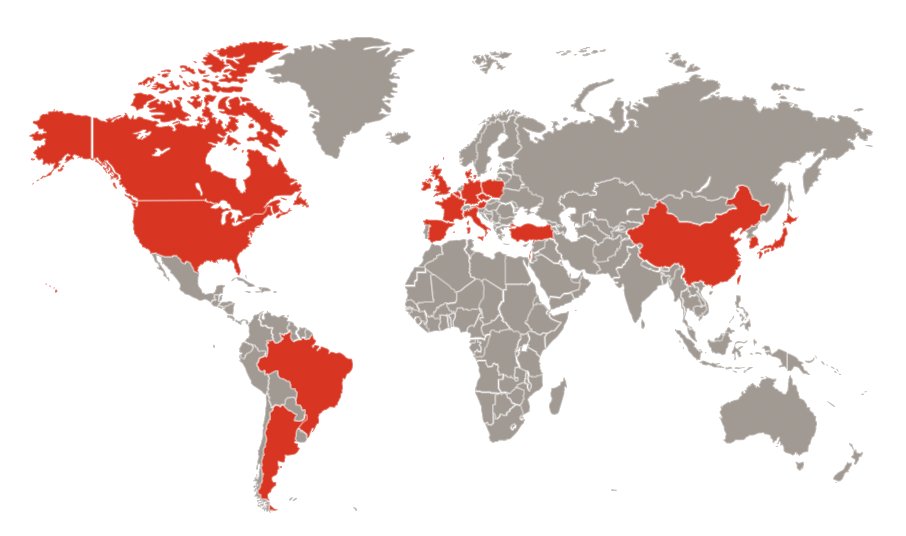
As of mid-April, enrollment is open at 3 sites in the United States and 1 in Israel.
Presented by: Roger Li, MD, Genitourinary Oncologist, Moffitt Cancer Center, Tampa, FL
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 - Mon, May 6, 2024.
Reference:
- Vilaseca A, et al. First safety and efficacy results of the TAR-210 erdafitinib (erda) intravesical delivery system in patients (pts) with non–muscle-invasive bladder cancer (NMIBC) with select FGFR alterations. Ann Oncol. 2023;34:S1343


