(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on bladder cancer trials in progress, and a presentation by Dr. Mark Tyson discussing BOND-003- Cohort P, a multi-national, single-arm study of intravesical cretostimogene grenadenorepvec for the treatment of high risk, papillary only, BCG-unresponsive non-muscle invasive bladder cancer. Cretostimogene Grenadenorepvec is a highly immunogenic conditionally replication adenovirus, and its oncolytic immunotherapy mechanism is that it encodes GM-CSF with insertion of the human EF2-1 promoter:
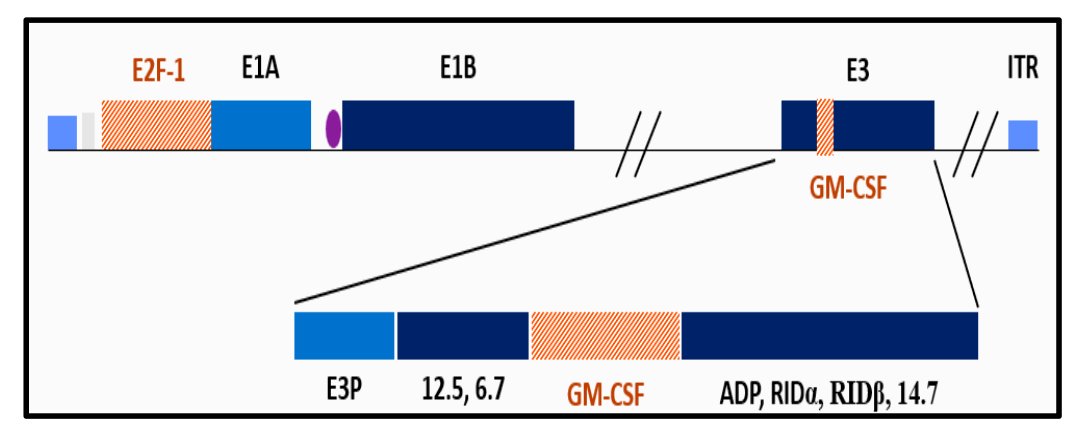
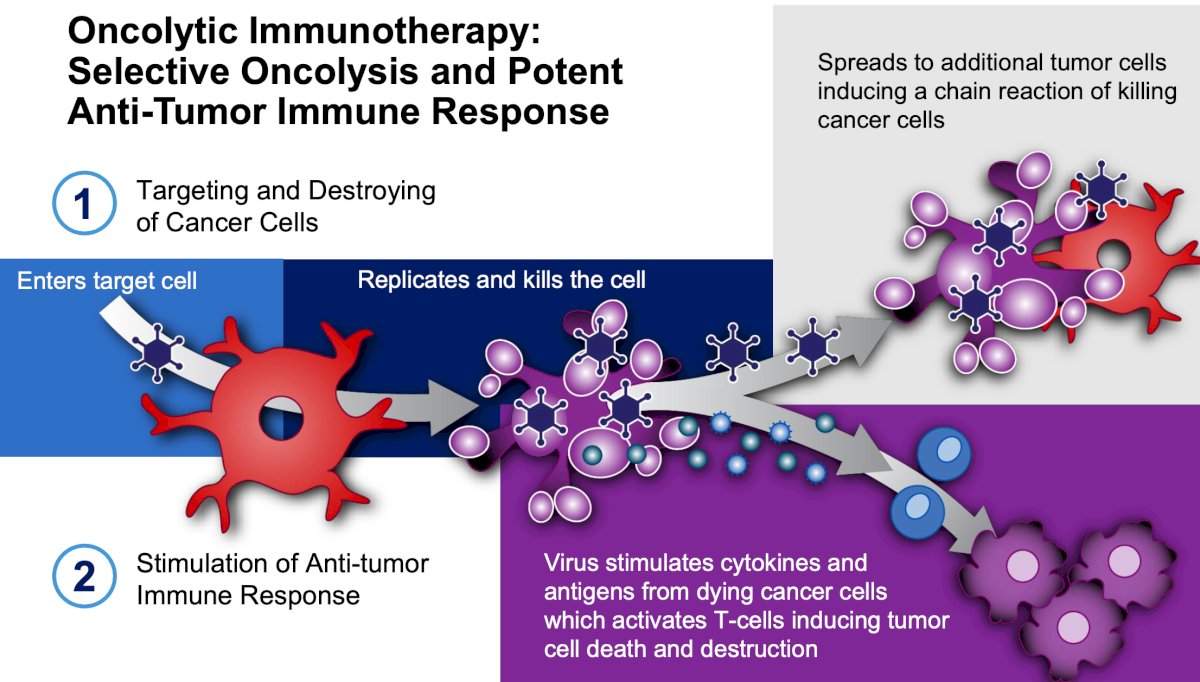
It binds to the Coxsackie Adenovirus Receptor (CAR) leading to robust expression in all stages of bladder cancer, and subsequent viral replication results in tumor lysis. Dr. Tyson notes that oncolytic immunotherapy is selective oncolysis and potent antitumor response, with the following steps:
- Targeting and destroying cancer cells
- Stimulation of anti-tumor response
The updated results from BOND-003 (n = 105) CIS +/- Ta/T1, presented at AUA 2024 by Dr. Tyson, included a 75.2% (95% CI 65% - 83%) complete response rate at any time based on central review. This is in the context of all patients having active disease at baseline prior to enrollment and having received adequate BCG therapy as per FDA 2018 Guidance on BCG-unresponsive NMIBC. Cretostimogene also showed a durable response over time:
- 53.8% of repeat induction patients converted to a complete response
- 52 patients have a duration of response >= 6 months
- 29 patients have a duration of response >= 12 months
- 14 patients have a duration of response >=21 months
- 92.4% cystectomy-free survival, with none of the patients with a complete response having undergone radical cystectomy, and none having nodal or metastatic progression
- 96.7% progression-free survival at 12 months
The Swimmer’s plot for the updated results is as follows:
Cretostimogene has been generally well tolerated, with most adverse events being grade 1-2, with 2 patients (1.8%) having serious treatment-related adverse events (grade 2). There were no grade >= 3 treatment-related adverse events reported, one patient with treatment discontinuation due to an unrelated adverse event, and 94.5% of patients completing all treatments:
Based on these and the initial results of BOND-003, the FDA has granted fast-track designation for cretostimogene monotherapy in BCG-unresponsive CIS with or without Ta/T1 papillary disease.
There is a significant unmet need for BCG unresponsive papillary nonmuscle invasive bladder cancer, given that there is a growing incidence of patients with papillary Ta/T1 disease. The unmet need is for highly effective, well-tolerated, readily available, and durable treatment options for patients with Ta/T1 papillary-only disease. BOND-003 Cohort P is for ~75 participants with Ta/T1 only disease to receive induction + maintenance with cretostimogene, with a primary endpoint of event-free survival: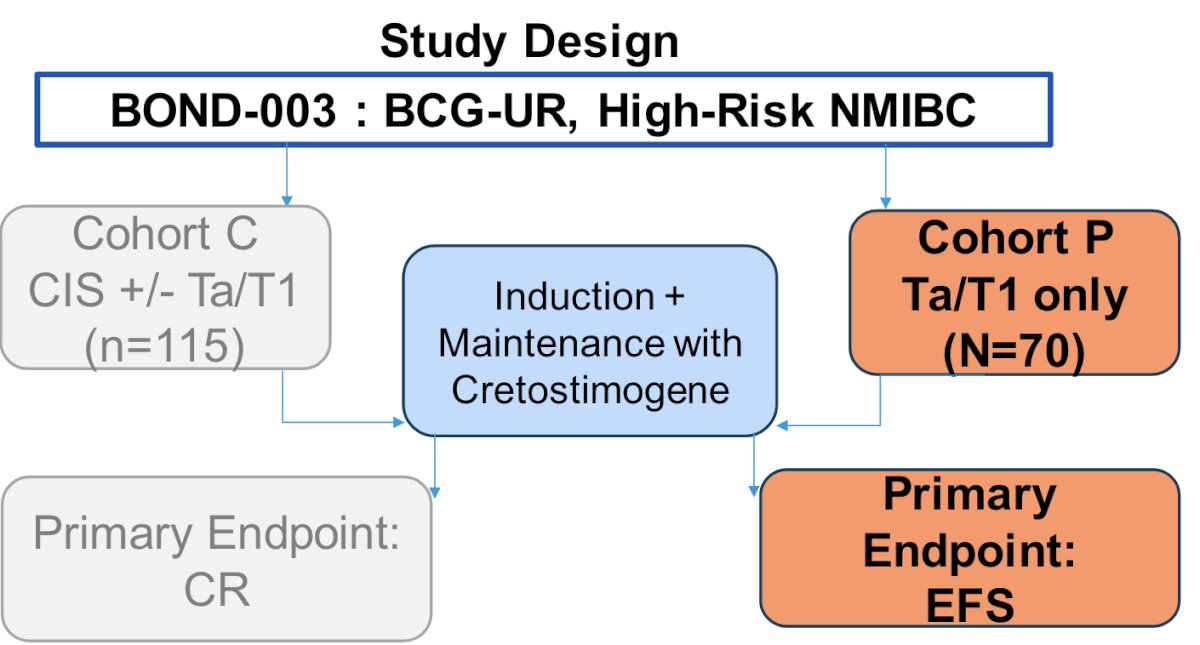
Dr. Tyson noted that cretostimogene has a familiar and convenient installation process/schedule for practices, with a thaw time of up to 10 minutes. It can be administered by allied healthcare professions and is generally well tolerated. 100% of patients in BOND-003 had a successful instillation, which will streamline use for future cohorts and studies.
For Cohort P, the first sites opened, and patients enrolled in March 2024, with an anticipated recruitment timeline of 12 months. The first 35+ sites were selected across North America and Japan, with further site selection ongoing.
Presented by: Mark Tyson II, MD, MPH, Urologic Oncologist, Mayo Clinic, Scottsdale, AZ
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 - Mon, May 6, 2024.


