(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on bladder cancer trials in progress, and a presentation by Dr. Robert Svatek discussing PIVOT-006, a phase 3 randomized study of adjuvant intravesical cretostimogene grenadenorepvec versus surveillance for the treatment of intermediate risk non-muscle invasive bladder cancer following transurethral resection of bladder tumor. Cretostimogene Grenadenorepvec is a highly immunogenic conditionally replication adenovirus, and its oncolytic immunotherapy mechanism is that it encodes GM-CSF with insertion of the human EF2-1 promoter:
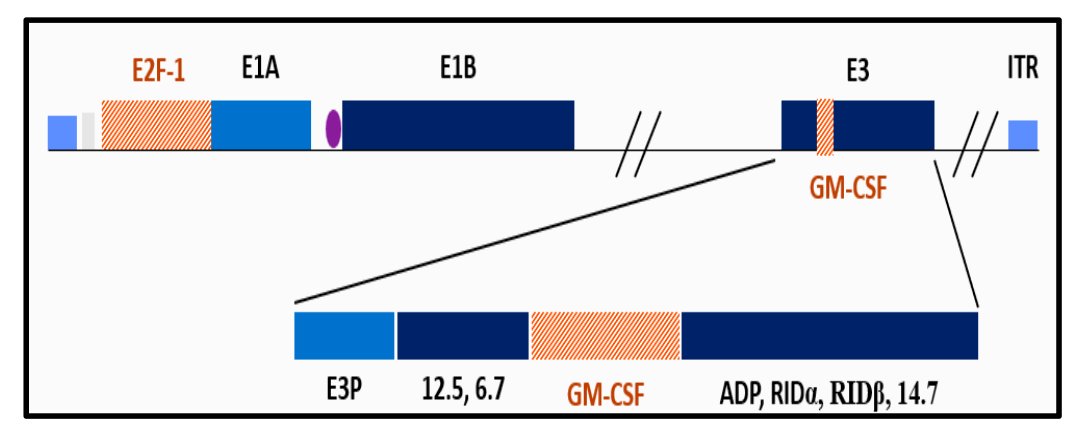
It binds to the Coxsackie Adenovirus Receptor (CAR) leading to robust expression in all stages of bladder cancer, and subsequent viral replication results in tumor lysis.
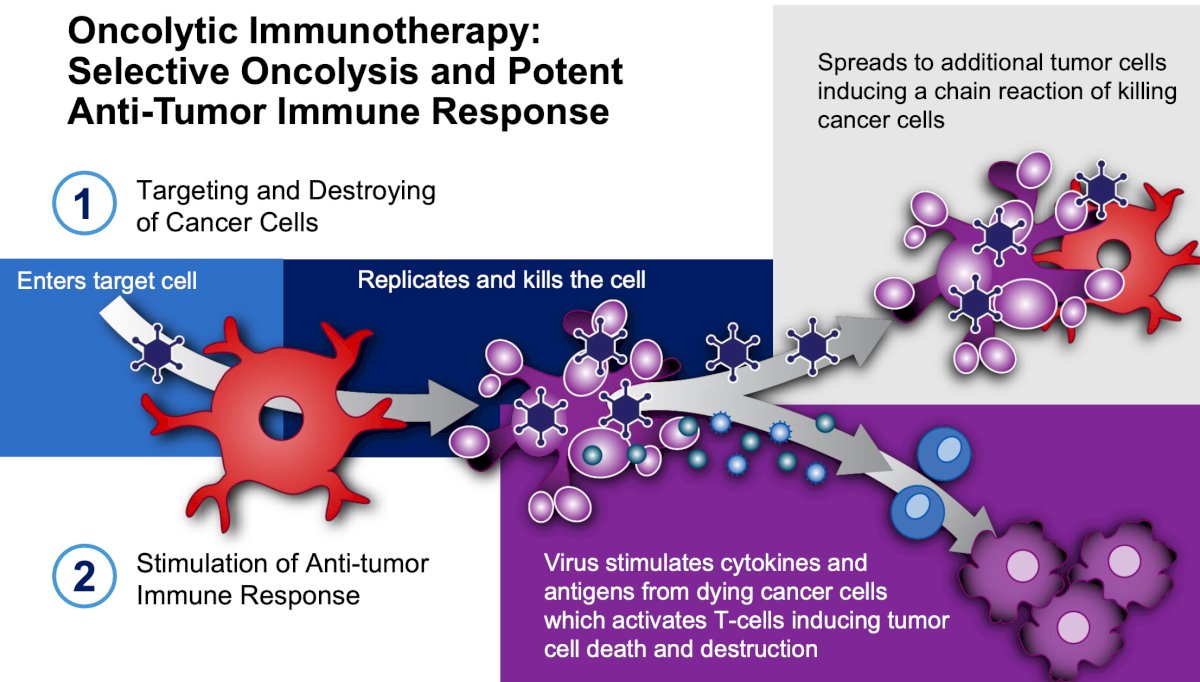
Dr. Svatek notes that oncolytic immunotherapy is selective oncolysis and potent antitumor response, with the following steps:
- Targeting and destroying cancer cells
- Stimulation of anti-tumor response
There is a significant unmet need with a high prevalence of intermediate-risk non-muscle invasive bladder cancer in the United States. At disease presentation, 75% have non-muscle invasive bladder cancer, of which there is the following breakdown:
- Low risk: ~30%
- Intermediate risk: ~30%
- High risk: ~40%
Intermediate risk non-muscle invasive bladder cancer has a high risk of recurrence (50%-70%), low risk of progression (<5%), is costly, common, chronic, and is associated with risks of anesthesia and surgery from multiple TURBTs.
The AUA risk stratification for intermediate risk disease is as follows:
- Low grade urothelial carcinoma:
- T1, or
- >3 cm, or
- Multifocal, or
- Recurrence with 1 year
- High grade urothelial carcinoma:
- Ta, and
- <=3 cm, and
- Solitary
According to the NCCN guidelines, the preferred treatment for intermediate risk NMIBC is intravesical therapy or surveillance. PIVOT-006 is a phase 3 open label, North American trial of adjuvant cretostimogene versus TURBT alone in intermediate risk NMIBC. The expected recruitment for this trial is up to 364 participants who will be randomized to either intravesical cretostimogene after TURBT versus TURBT alone. The key inclusion criteria are:
- Intermediate risk NMIBC per the AUA/SUO 2020 definition which is pathologically confirmed within 12 weeks of the study start
- All visible disease removed by TURBT within 12 weeks of randomization
- 18 years of age or older
- ECOG performance status 0-2
- Willing to use barrier contraception 14 days prior to and 6 weeks post cretostimogene doses
- Willing to comply with study mandated procedures
- Prior high grade NMIBC is permitted if intermediate NMIBC is present at study entry
- Prior BCG and intravesical chemotherapy is permitted, after a washout period
The key exclusion criteria are:
- Current or prior evidence of high risk NMIBC (high grade T1, high grade Ta >3 cm that is recurrent or multifocal, any CIS, any variant histology, any high grade prostatic urethra involvement, any LVI, any BCG treatment failure in high grade patients)
- Has intermediate risk NMIBC that cannot be completely resected
- Low risk NMIBC (low grade solitary Ta <= 3 cm that has recurred more than 12 months after a previous high grade or low grade tumor). No low grade, low risk disease
- Has a history of current muscle invasive bladder cancer (T2 or higher stage), locally advanced (T3/T4, any N) or metastatic disease
Stratification factors will be receipt of perioperative chemotherapy and high versus low grade intermediate risk disease. Disease assessment will be an every 3 month cystoscopy and urine cytology for 2 years and then every 6 months starting in year 3. Imaging will be every 12 months and pathology will be centrally reviewed. Importantly, control arm patients will be offered cretostimogene treatment following intermediate risk NMIBC recurrence. The trial schema for PIVOT-006 is as follows:
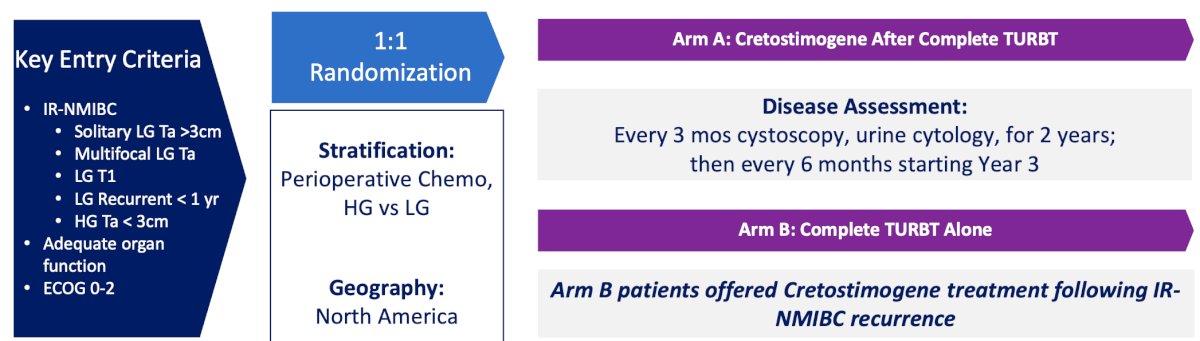
The key endpoints for the trial are:
- Recurrence free survival (without recurrence or progression in the bladder, upper tract or prostatic urethra)
- Recurrence free survival at 12 months and 24 months
- Progression free survival
- Safety
There has been tremendous support for the PIVOT-006 trial, including support from the SUO-CTC, BCAN, and Parexel Biotech. Currently, there is over 90 North American sites that are participating in the trial, with the first enrolled patient in January 2024. The expected enrollment period is 30 months, with at least 1 year of follow-up (NCT06111235)
Presented by: Robert Svatek, MD, UT Health San Antonio, San Antonio, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
Related content: PIVOT-006: Phase 3 Trial Comparing Oncolytic Virus Creto vs Observation for Intermediate-Risk NMIBC - Robert Svatek


