(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX was host to the Bladder Cancer Non-invasive podium session. Dr. Vikram Narayan presented the final results from the 60-month follow-up of the phase 3 trial evaluating the efficacy of intravesical Nadofaragene Firadenovec-vcng for patients with Bacillus Calmette-Guerin (BCG)-unresponsive carcinoma in situ (CIS) of the bladder.
Patients with BCG-unresponsive non-muscle invasive bladder cancer (NIMBC) remain at significant risk for disease recurrence and progression. Bladder preserving strategies for patients with BCG unresponsive disease are limited and radical cystectomy remains the guideline-recommended, standard of care treatment for such patients.
Nadofaragene firadenovec (Adstiladrin®) is a novel replication-deficient recombinant adenovirus vector-based gene therapy that delivers human interferon alpha-2b to urothelial cells. Adstiladrin® has been approved by the United States Food and Drug Administration (FDA) for the treatment of BCG-unresponsive NMIBC carcinoma in situ (CIS) with/without papillary tumors (± Ta/T1) (Figure 1).
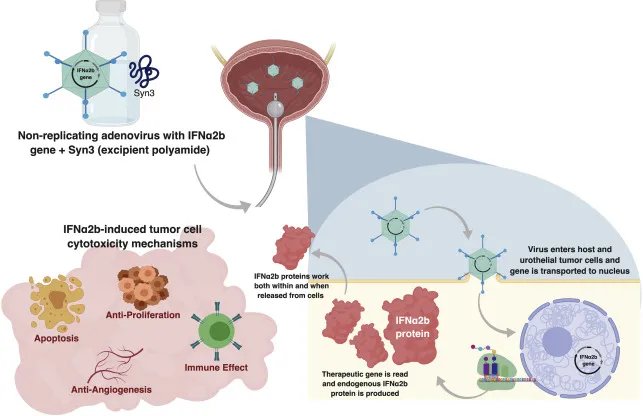
In 2021, Boorjian et al. published the primary efficacy, safety, and durability outcomes of the phase 3 trial of Nadofaragene Firadenovec in patients with BCG-unresponsive NMIBC. Initially, 53.4% of patients with CIS +/- Ta/T1 papillary disease had a complete response within 3 months of the first dose, and this response was maintained in 46% of patients at 12 months. The objective of this presentation was to report the final outcomes from the 60-month follow-up including high-grade recurrence-free survival, cystectomy-free survival, and overall survival.1
This was a single arm, open-label, multicenter phase 3 trial (NCT02773849) that enrolled patients with BCG-unresponsive NMIBC in two cohorts:
- Cohort 1: CIS +/- Ta/T1 disease (n=107)
- Cohort 2: Ta/T1 without CIS (papillary disease) (n=50)
The treatment protocol of this trial involved administering 75 ml of nadofaragene firadenovec (3 x 1011 viral particles/mL) once every three months for up to four doses. Per protocol, a 5-site biopsy (dome, trigone, right and left lateral walls, and posterior wall) was performed at 12 months, and patients who were high-grade recurrence-free were offered continued treatment at the investigator’s discretion. Assessments beyond 24 months were performed in accordance with usual clinical practice. This report is based on the 60-month data for both cohorts. The figure below shows the trial design.
For all treated patients (n=157), the median follow-up was 50.8 months (IQR: 39.1, 60.0) with 26.8% of patients receiving 5 or more instillations and 7.6% of patients receiving treatment for at least 57 months.
In the efficacy analysis set the investigators share the 5-years outcomes of interest, including high-grade recurrence-free survival (HG-RFS), cystectomy-free survival, and overall survival:
- High-grade recurrence free survival: 5.8% of patients with CIS +/- Ta/T1 (6/103) were HG-recurrence free at 57 months, and 14.6% (7/48) of patients with PD (Ta/T1) were HGRF at month 57. Kaplan-Meier-estimated HG-RFS survival rate at 57 months was 13.2% and 32.7% in the CIS (CIS +/- Ta/T1) and PD (Ta/T1) cohorts, respectively.
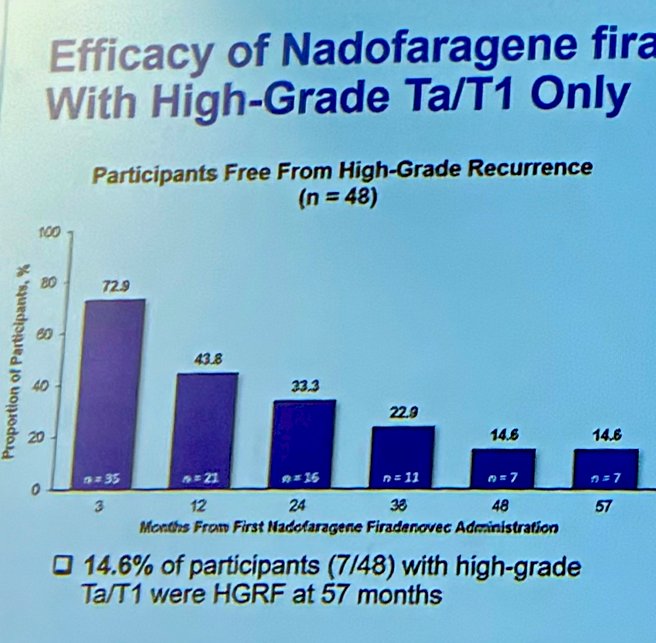
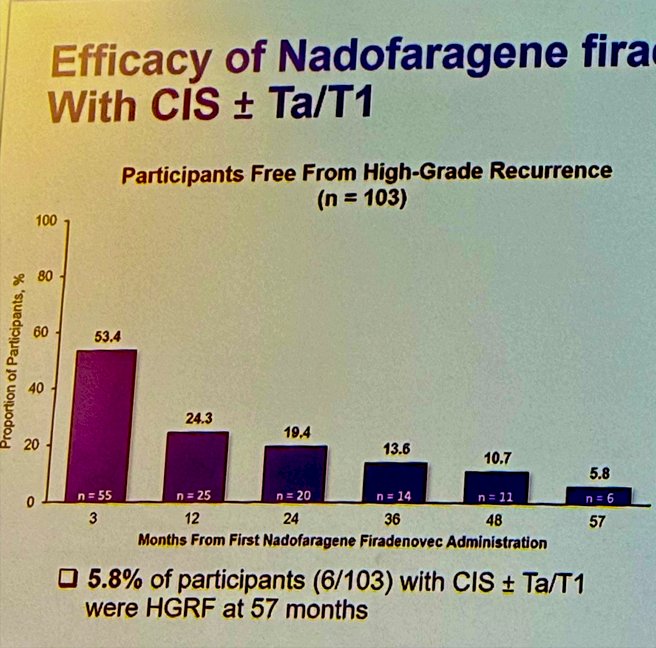
The duration of high-grade RFS in participants with PD (Ta/T1 only) was explored, 17/35 (48.5%), and 14/55 (25.4%) of the CIS +/- Ta/T1 cohort had an ongoing response at the time of data analysis.
The 5-year cystectomy-free survival (CFS) was also measured, starting after the first dose of nadofaragene firadenovec, the 60-month CFS in the CIS cohort was 43.2% and 58.7% in the PD cohort and the overall 5-year CFS was 48.8%. In terms of overall survival (OS), At 60 months THE OS was 76.3% (64.6, 84.5) and 85.9% (70.9, 93.5) in the CIS and PD cohorts, respectively.
Four patients with CIS and one patient with PD experienced progression to muscle-invasive disease documented by transurethral resection of bladder tumor at the time of high-grade recurrence.
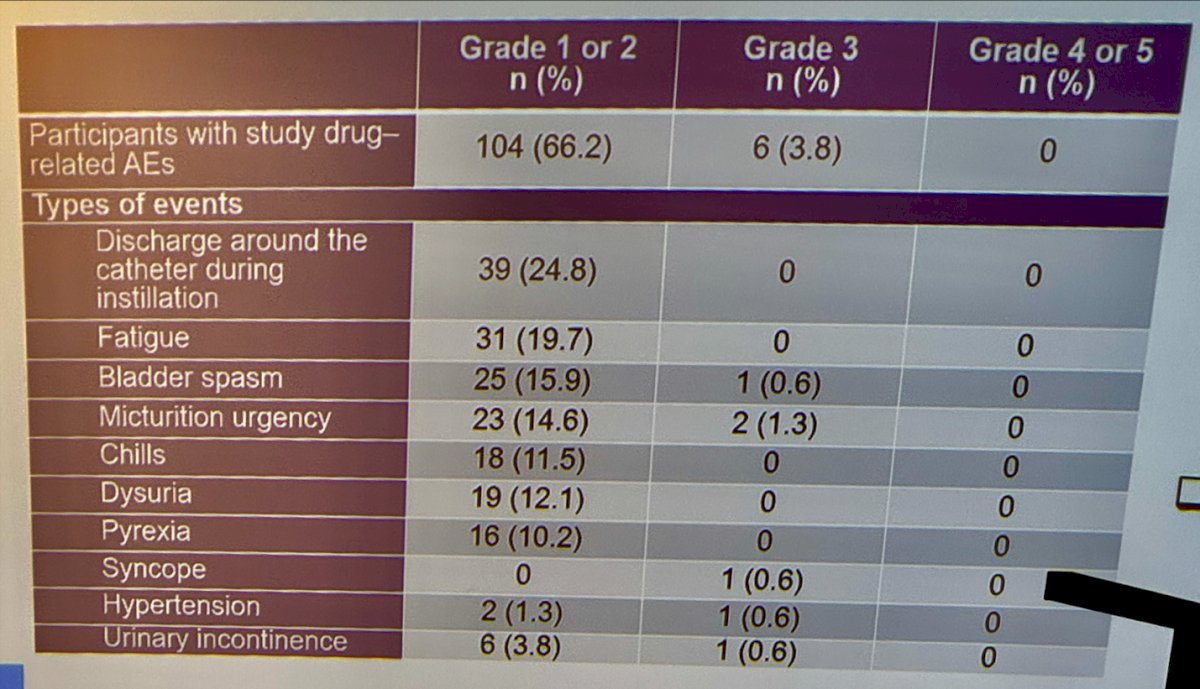
No new safety signals were reported by the investigators on long-term follow-up (60 months). They reported 66% of the patients endorsed Grade1/2 treatment related adverse events (TRAEs) and 3.8% experienced Grade 3/4 TRAEs. The most common adverse event was discharge around the catheter during instillation reported in 24.8%. Other TRAEs are shown in the table below.
Dr. Narayan concluded with the following key takeaways:
- Intravesical nadofaragene firadenovec, administered once every 3 months, demonstrated a sustained durability of initial complete response in approximately 25% of patients with BCG-unresponsive CIS +/- Ta/T1 papillary disease and 48.5 % in Ta/T1 patients through 60 months
- Nadofaragene firadenovec provided nearly half of the participants with bladder preservation at 60 months.
- The study drug was well tolerated with no study TRAEs grade 4/5 and no new safety signals in the long-term.
- Nadofaragene firadenovec represents a safe and novel intravesical treatment option for BCG-unresponsive NMIBC and no new safety signals on long-term follow-up were identified.
Presented by: Vikram Narayan, MD, Urologist at Emory University, Atlanta, GA
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:


