(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on non-invasive bladder cancer, and a presentation by Dr. Mohamad Abou Chakra discussing the results of a survey on clinical practice patterns in the United States for using intravesical chemotherapy in the management of BCG unresponsive non-muscle invasive bladder cancer. It is unknown how patients with non-muscle-invasive bladder cancer who are BCG-unresponsive are treated in a real-world setting. When giving intravesical chemotherapy to these patients, it is critical to understand the therapeutic choices that are made and if the selection of therapy is dependent on tumor type (papillary versus CIS). The goal of this survey was to evaluate the treatment and practice patterns of urologists treating high-grade papillary-only tumors and CIS in the BCG-unresponsive setting.
For this study, US urologists who treat non-muscle-invasive bladder cancer cases were provided with a 5-minute online survey to complete. A target list of respondents was established based on their high BCG use. One survey, which was conducted from January 6–19, 2022, inquired about the treatment given to patients with CIS. The second survey was active from April 12–18, 2022, and inquired about the treatment for patients with papillary-only tumors. The use of intravesical chemotherapy in BCG-unresponsive cases was the main focus of both surveys. Each urologist’s regimens of chemotherapy were recorded when intravesical chemotherapy was utilized.
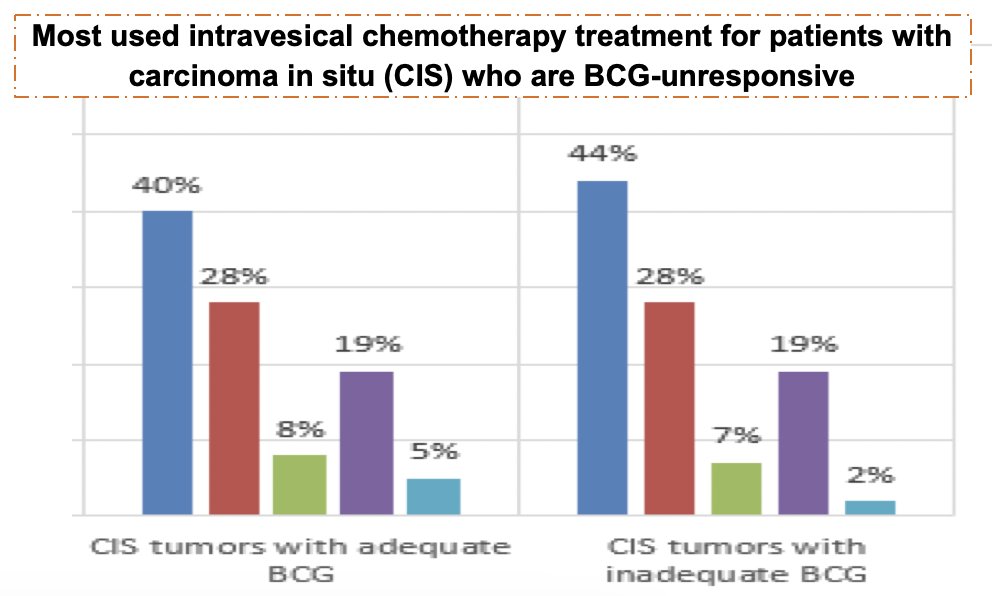
The survey was completed by 159 and 100 urologists who managed CIS and papillary-only tumors, respectively. The majority (78%) were community-based, and slightly more than half reported that chemotherapy was their preferred course of treatment, followed by radical cystectomy (one third) and systemic immune therapy (15%). For perceived clinical benefit, the median 1-year minimum effectiveness was 35%. Overall, 40% gemcitabine, 28% mitomycin-C, 19% valrubicin, 8% gemcitabine/docetaxel, and 5% others were the rank order for CIS patients with adequate BCG:
The rank order was slightly different for BCG-unresponsive papillary-only disease: gemcitabine 49%, mitomycin-C 23%, gemcitabine/docetaxel 15%, valrubicin 11%, and other 2%:
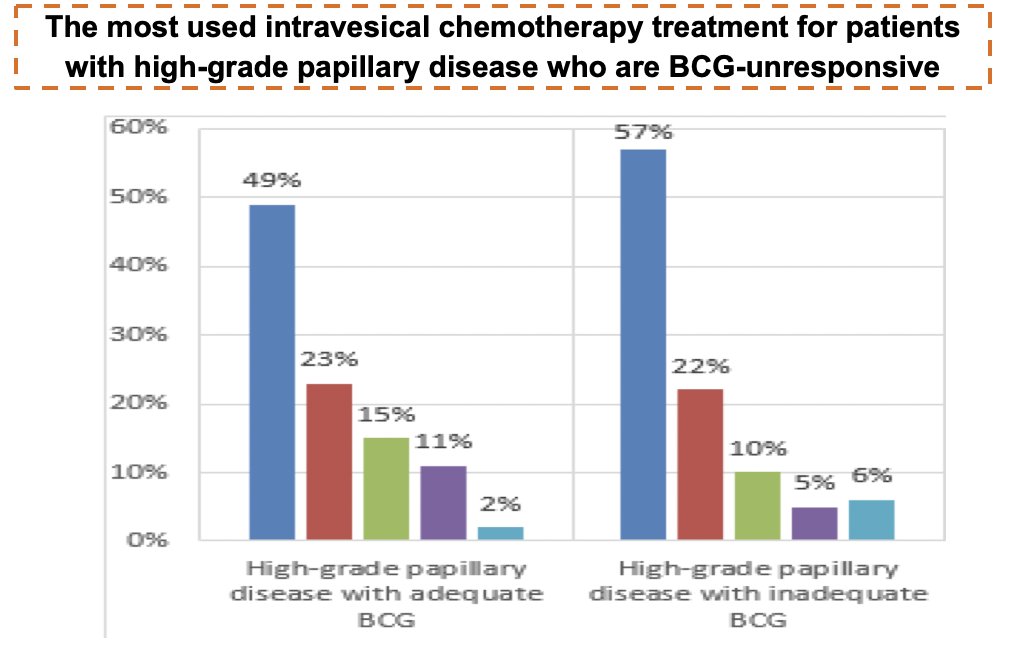
For CIS and papillary tumors with inadequate BCG exposure, a very similar profile was observed for adequate exposure in each type.
Dr. Abou Chakra concluded his presentation by discussing the results of a survey on clinical practice patterns in the United States for using intravesical chemotherapy in the management of BCG unresponsive non-muscle invasive bladder cancer with the following take-home messages:
- This recent poll of US urologists revealed that intravesical single-agent chemotherapy was the most often recommended treatment for BCG-unresponsive non-muscle-invasive bladder cancer, with gemcitabine or mitomycin-C making up about 70% of the chemotherapy regimens
- Whether therapy was for papillary-only or CIS tumors, regardless of BCG exposure, this practice pattern remained consistent
Presented by: Mohamad Abou Chakra, MD, University of Iowa, Iowa City, IA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


