(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured the AUA-IBCG Bladder Cancer Forum and a series of presentations by Drs. Jeremy Teoh, Neal Shore, and Badrinath Konety discussing whether skill urologists really need to perform re-TUR for all high-grade patients. As moderator, Dr. Shore started by presenting a case of a 65-year-old patient that presented with a solitary 2 cm papillary lesion with surrounding erythema on the lateral wall. His medical history included a two pack per day smoker for 15 years (quit 15 years ago), but he is now vaping, and he is 20 kg overweight. He previously worked in a steel mill and is now a compliance officer for a pharmaceutical company. He eventually underwent a complete resection TURBT, but had a thin bladder wall, as well as a difficult post-anesthesia recovery. Dr. Shore polled the audience with the following question: “Do skilled urologists really need to perform a re-TUR for all high grade patients?” with the following results:
- Yes: 28%
- Only T1: 55%
- No: 17%
Dr. Teoh started by taking the stance of no need for repeat TURBT. From a surgical perspective, CIS and early cancer changes can be subtle, it can be difficult to determine the resection margin based on white light imaging alone, and difficult to ensure a uniform resection at the detrusor muscle level. Thus, the end result is residual disease. According to the AUA guidelines specific to a repeat TURBT, the following statements are made:
- Guideline statement 12: In a patient with non-muscle invasive disease who underwent an incomplete initial resection (not all visible tumor treated), a clinician should perform repeat transurethral resection or endoscopic treatment of all remaining tumor if technically feasible. (Strong Recommendation; Evidence Strength: Grade B)
- Guideline Statement 13: In a patient with high-risk, high-grade Ta tumors, a clinician should consider performing repeat transurethral resection of the primary tumor site within six weeks of the initial TURBT. (Moderate Recommendation; Evidence Strength: Grade C)
- Guideline Statement 14: In a patient with T1 disease, a clinician should perform repeat transurethral resection of the primary tumor site to include muscularis propria within six weeks of the initial TURBT. (Strong Recommendation; Evidence Strength: Grade B)
Dr. Teoh raised several important questions, including (1) Why do we need a second surgery to compensate for the first surgery? (2) Why do we think that a second surgery would be easier than the first surgery? With en bloc resection of specimens, both circumferential and deep resection margins can be inked and assessed, as well as the depth of tumor invasion be ascertained. Importantly, proper staging and complete tumor resection can be achieved:

Dr. Teoh was the lead author of a recently published phase 3 randomized trial assessing transurethral en bloc resection versus standard resection of bladder tumors.1 The EB-StaR study was a multicenter, randomized, phase 3 trial conducted in Hong Kong. There were 350 patients with bladder tumor(s) of ≤3 cm recruited from April 2017 to December 2020 and followed up until 1 year after surgery. The primary outcome was 1-year recurrence rate. Overall 276 patients were confirmed to have non-muscle invasive bladder cancer, including 143 patients in the en bloc resection group and 133 patients in the standard resection group. At 1 year, 31 patients in the en bloc resection group and 46 in the standard resection group developed recurrence. The Kaplan-Meier estimate of 1-year recurrence rates was 29% (95% CI 18–37) in the en bloc resection group and 38% (95% CI 28–46) in the standard resection group (p = 0.007):
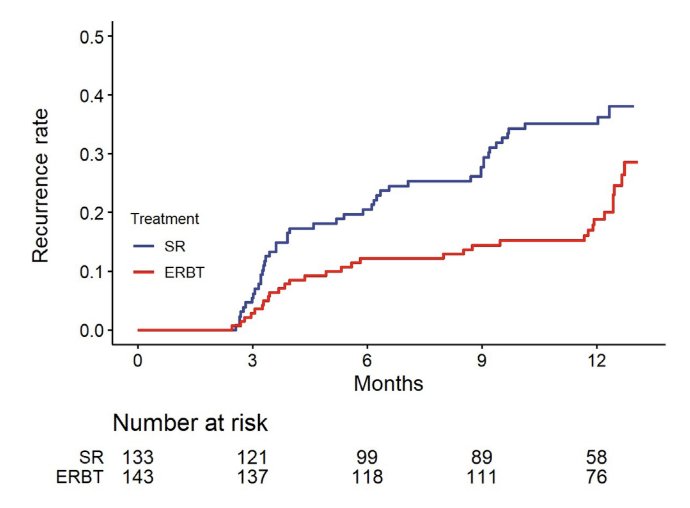
The forest plot shows that patients with 1-3 cm tumors, single tumors, Ta disease, or intermediate risk non-muscle invasive bladder cancer had a significant benefit from en bloc resection:
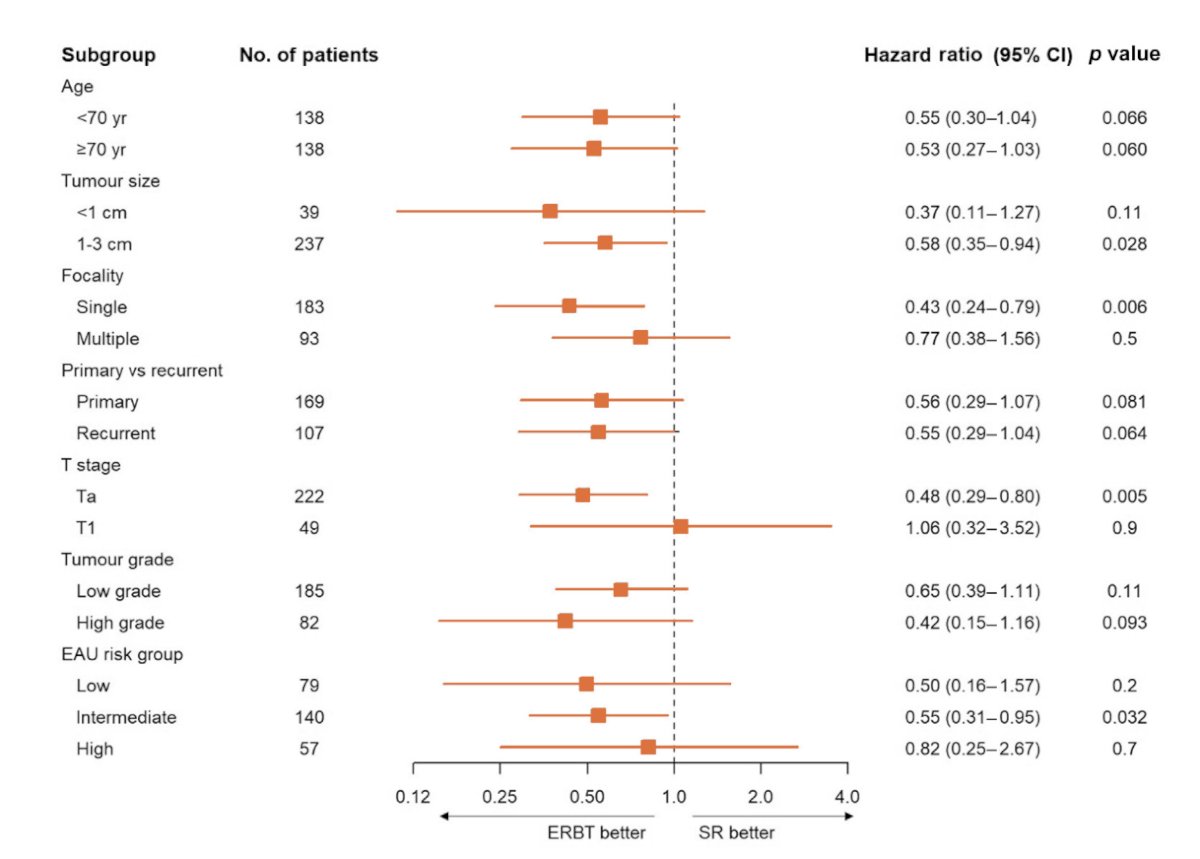
Dr. Teoh notes that the modified ERBT study is a phase 2 single arm study using modified en block resection. Key inclusion criteria include bladder tumors >= 3 cm, and assuming a >90% rate of complete resection for non-muscle invasive bladder cancer and proper staging for muscle invasive bladder cancer, 30 patients were to be recruited. The primary outcome is complete resection and proper staging of muscle invasive bladder cancer, and the study is registered at clinicaltrials.gov (NCT04081246). One case example from this trial is as follows:
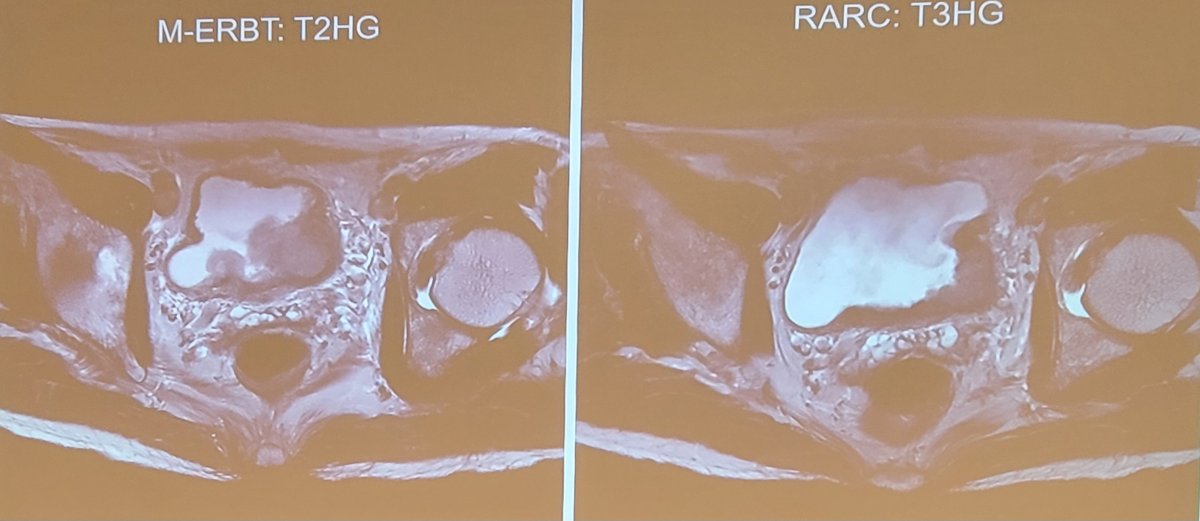
Among 28 cases of modified en bloc resection, one case of prostate cancer was excluded, finding 15 cases of non-muscle invasive bladder cancer (with 14 cases of complete resection – 93.3%), and 12 cases of muscle invasive bladder cancer (11 had proper staging of muscle invasive disease at the first surgery – 91.7%). Ultimately 8 patients underwent robotic radical cystectomy, with four patients having T2 disease ultimately having T0 disease on final pathologic staging. The remaining four patients had pT3 disease. Thus, Dr. Teoh notes that modified en bloc resection achieved complete resection of non-muscle invasive bladder cancer and proper staging of muscle invasive bladder cancer in 92.6% of cases.
Dr. Teoh concluded his portion of the debate with the following thoughts:
- Conventional TURBT is associated with major limitations
- Second/repeat TURBT is often offered to compensate for the first surgery
- In the modern era, with the advancement of MRI, enhanced imaging, and newer surgical approaches, should second/repeat TURBT still be offered?
- A good surgery is the first and the most crucial step to cure non-muscle invasive bladder cancer and possibly a major step towards bladder sparing approaches for muscle invasive disease
Dr. Konety then took the position of yes, all patients should receive a repeat TURBT. The following guidelines offer recommendations for repeat TURBT:
- AUA:
- Incomplete resection (Grade B)
- All T1 lesions (Grade B)
- Recurrence high grade Ta lesions (Grade C)
- ICUD:
- Incomplete initial resection
- T1 high grade disease after a complete initial resection
- High grade Ta tumors, particularly those that are large or multifocal
- EAU (Grade A):
- Incomplete resection
- Absence of muscle on the first resection (except CIS/low grade Ta)
- All high grade Ta and T1 lesions
- NCCN:
- Incomplete resection
- T1
- Large and multifocal
- No muscle in the initial specimen with high grade Ta
The quality of TURBT likely is related to the person performing the TURBT. Previous studies suggest that tumor at the time of re-TURBT is 4x higher with residents versus expert consultants. Additionally, seminal work from Dr. Harry Herr previously noted that on re-TURBT, 29% of non muscle invasive bladder cancer was upstaged, and 22% of muscle invasive bladder cancer was downstaged. A subsequent systematic review from Cumberbatch et al.2 identified 31 studies including 8,409 patients with high grade Ta and T1 bladder cancer. Detrusor muscle was found at initial TURBT histology in 30-100% of cases, and residual tumor at re-TURBT was found in 17-67% of patients following Ta and in 20-71% following T1 cancer. Most residual tumors (36-86%) were found at the original resection site, and upstaging occurred in 0-8% (Ta to ≥T1) and 0-32% (T1 to ≥T2) of cases. There was no clear relationship between re-TURBT and progression for Ta disease, although T1 rates were higher in the non-re-TURBT group in series with control populations. Overall mortality was slightly reduced in the re-TURBT group in two studies with controls (22-30% vs 26-36% no re-TURBT).
Dr. Konety notes that a previous randomized controlled trial from 2010 published updated findings in 2020 with long term follow-up.3 This trial randomized 210 patients with stage pT1 non-muscle invasive bladder cancer who underwent a first TURBT into two groups including second TURBT (n = 105) and no second TURBT (n = 105). Over a median follow-up of 119 months (IQR 65-168), compared to patients without second TURBT, patients with second TURBT had significantly higher 5-year, 7-year and 10-year rates for recurrence free survival (59.4%, 57.9%, and 54.8% vs. 36.3%, 31.7%, and 26.8%, respectively, p < 0.001) and progression free survival (93.3%, 91.9% and 90.4% vs. 74.0%, 71.4% and 68.5%, respectively, p < 0.001):

Is a restaging TURBT necessary in high-risk NMBIC if the initial TURBT was performed with blue light? Dr. Konety was part of a study that sought to answer this question using a multi-institutional Cysview registry between 2014 and 2021 of all consecutive adult patients with known non-muscle invasive bladder cancer (Ta and T1 disease) who underwent TURBT followed by a restaging TURBT within 8 weeks.4 Overall, 115 patients had TURBT followed by a restaging TURBT within 8 weeks of which those that underwent blue light compared to white light for their initial TURBT had higher rates of benign pathology on restaging TURBT, although this was not statistically significant (47% vs. 30%; p = 0.08). Of patients with residual tumors on restaging TURBT, there were no differences in rates of Ta (22% vs. 26.5%; p = 0.62), T1 (22% vs. 26.5%; p = 0.62), or CIS (5.5% vs. 13%; P = 0.49) when the initial TURBT was done using blue light compared to white light. As follows are the rates of upstaging on re-TURBT stratified by initial pathology:
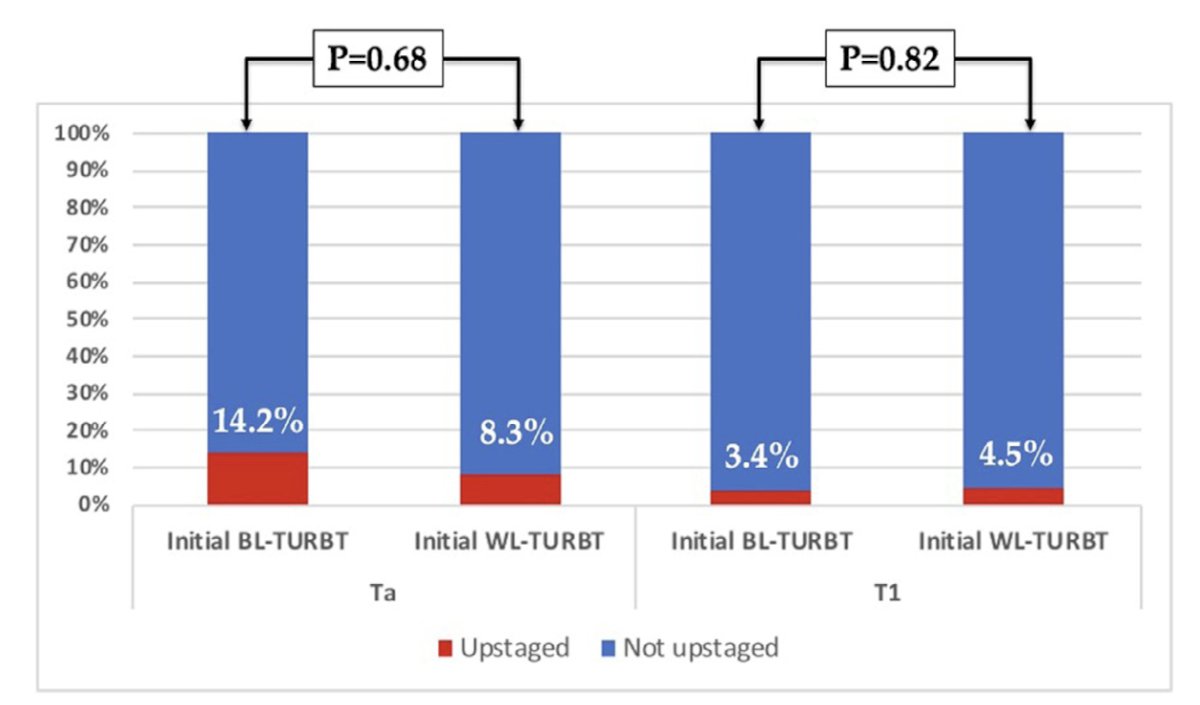
Historical studies also suggest that re-TURBT also predicts response to BCG, with residual tumor on re-TURBT being a key factor for recurrence (none: 11% vs residual: 28%) and progression (none: 5% vs residual: 17%).
Dr. Konety concluded his portion of the debate supporting re-TURBT with the following conclusions:
- There is more complete information up front with re-TURBT
- There is better patient selection for BCG
- It drives better cystectomy decision making
- It improves disease related outcomes
- It may improve overall survival
- En bloc resection samples the detrusor muscle in small tumors, but if what if there is no detrusor? It does not solve the issue if there is tumor found on re-TURBT
Moderator: Neal Shore, MD, FACS, Caroline Urologic Research Center, Myrtle Beach, SC
Debaters:- Jeremy Teoh, MBBS, FRCSEd, FCSHK, FHKAM, Chinese University of Hong Kong, Hong Kong
- Badrinath Konety, MD, Allina Health Cancer Institute, Minneapolis, MN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Teoh JYC, Cheng CH, Tsang CF, et al. Transurethral en bloc resection versus standard resection of bladder tumour. A randomized, multicentre, phase 3 trial. Eur Urol. 2024 April 30 [Epub ahead of print].
- Cumberbatch MGK ,Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: A systematic review. Eur Urol. 2018 Jun;73(6):925-933.
- Eroglu A, Ekin RG, Koc G, et al. The prognostic value of routine second transurethral resection in patients with newly diagnosed stage pT1 non-muscle-invasive bladder cancer: Results from randomized 10-year extension trial. Int J Clin Oncol. 2020 Apr;25(4):698-704.
- Alsyouf M, Ladi-Seyedian SS, Konety B, et al. Is a restaging TURBT necessary in high-risk NMBIC if the initial TURBT was performed with blue light? Urol Oncol. 2023 Feb;41(2):109.e9-109.e14.


