(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to a non-invasive bladder cancer podium session. Dr. Amanda Myers presented the results of a single-center analysis evaluating response rates to continued BCG in patients with BCG-exposed non-muscle invasive bladder cancer (NMIBC).
BCG remains the most effective treatment for patients with high-grade NMIBC. Clinical trials are designed around ‘categories’ of recurrences after BCG therapy, and the current trial design is based on historical response rates in BCG-naïve patients that may result in underpowered studies. The objective is thus to report response rates to additional BCG in patients who meet the criteria for BCG-exposed and BCG-unresponsive disease.
This was a single-center analysis of patients with high-grade recurrences after BCG who received additional BCG as primary therapy between January 2000 and September 2021. Recurrences >2 years following BCG were treated as new index cases. The Kaplan-Meier method was used to estimate survival outcomes. BCG-unresponsiveness was defined per the FDA criteria. BCG-exposed was defined per the International Bladder Cancer Group (IBCG) criteria, as high-grade recurrence within two years of BCG, but not meeting unresponsiveness criteria.
The study flow chart is illustrated below. Of 857 patients who received BCG, 213 experienced high-grade NMIBC recurrences. Twenty-five patients with concurrent upper tract disease were excluded, as these patients are not typically included in clinical trials. Of these 213 patients, 88 received additional BCG. Of these 88, 52, and 36 were classified as BCG exposed and unresponsive, respectively.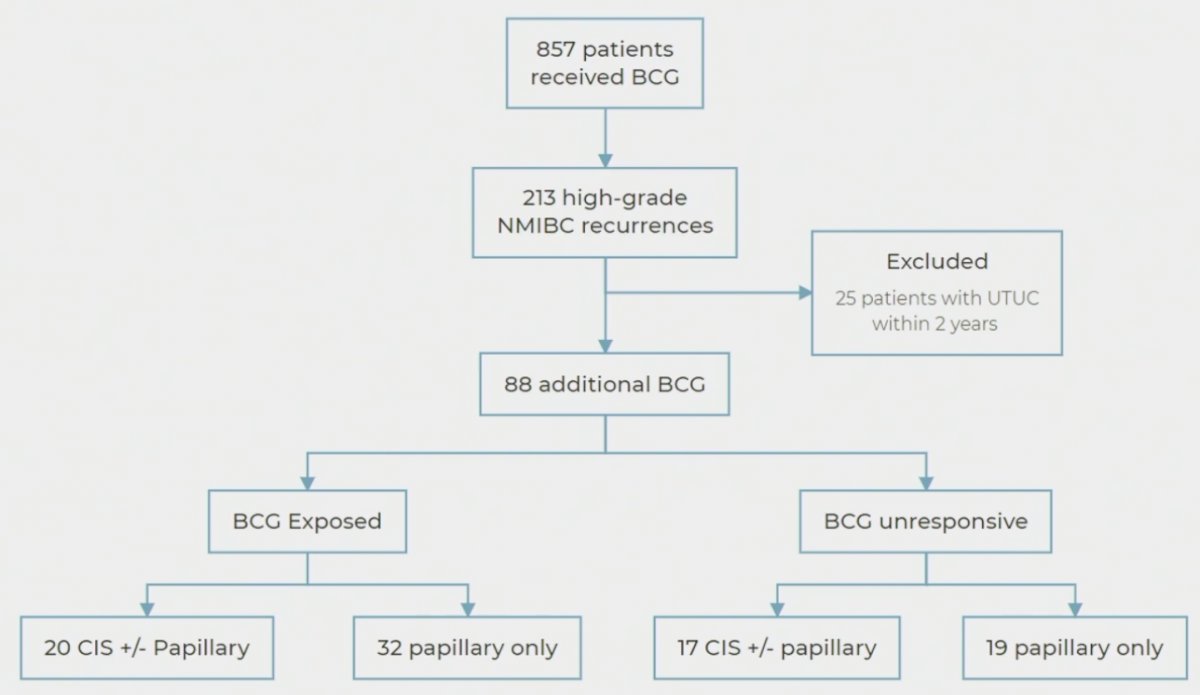
Summarized below are the tumor characteristics of BCG-exposed patients. Of these 52 patients, 46% received repeat induction BCG and 54% maintenance BCG. 79% of patients had no disease after additional BCG. The median duration of response was 159 months. 95% of responders were receiving ongoing maintenance BCG.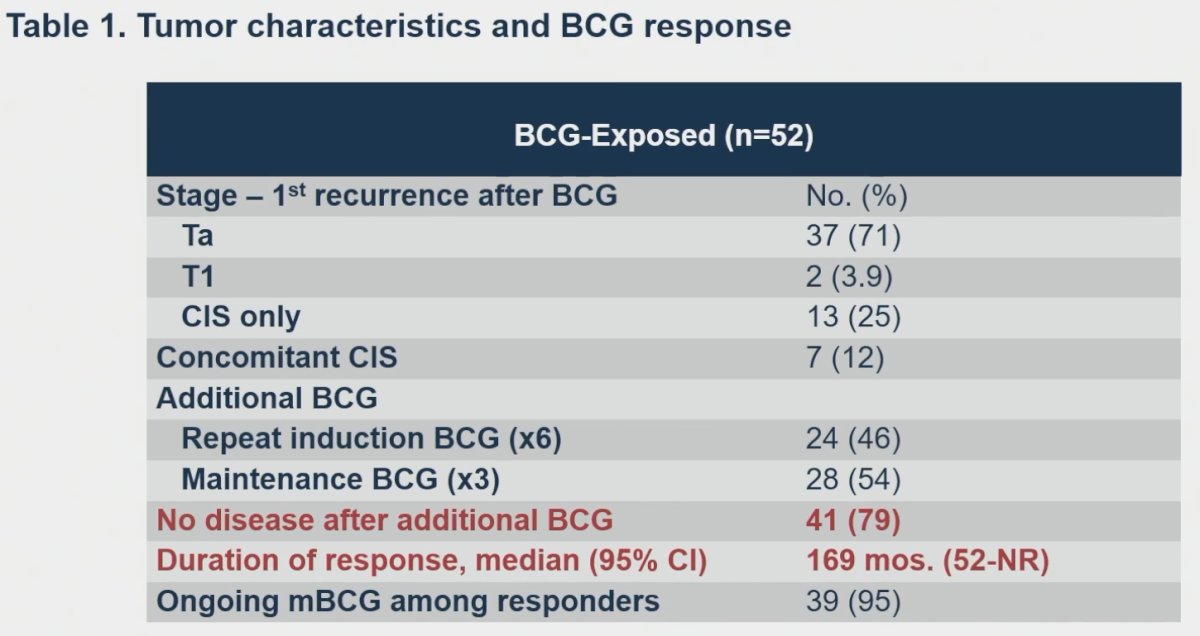
In BCG unresponsive patients, no disease following additional BCG was noted in 75% of patients, with a median duration of response of 83 months.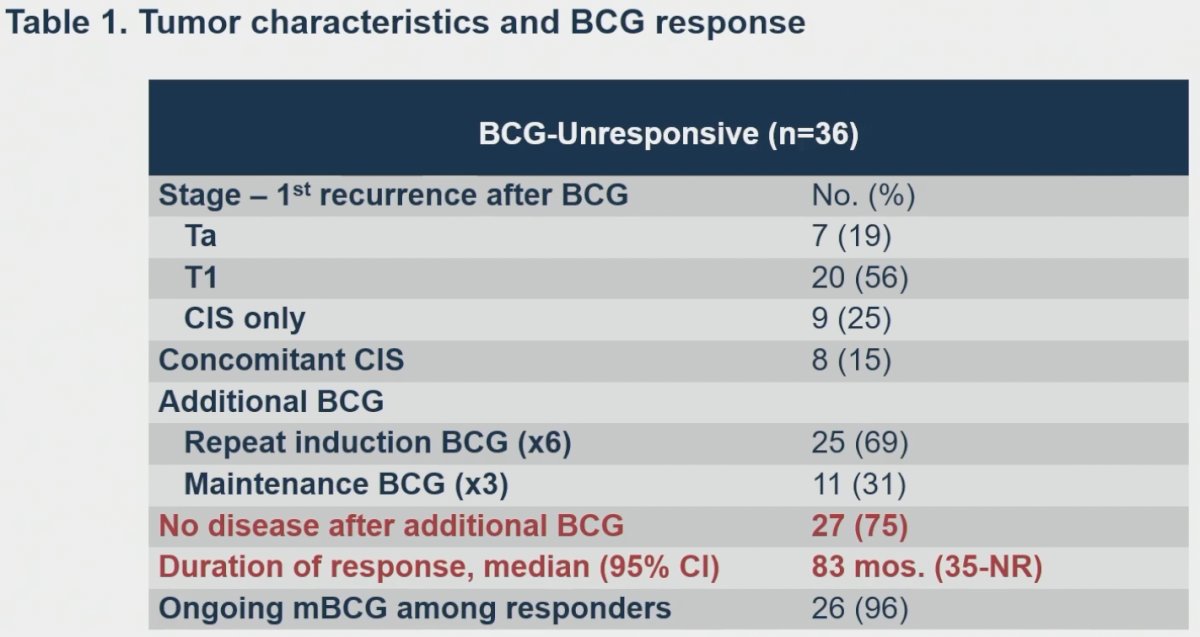
Two-year high-grade disease-free survival was 63% in both BCG-exposed and unresponsive patients.
Two-year progression-free survivals were 90% and 86% for BCG-exposed and unresponsive patients, respectively.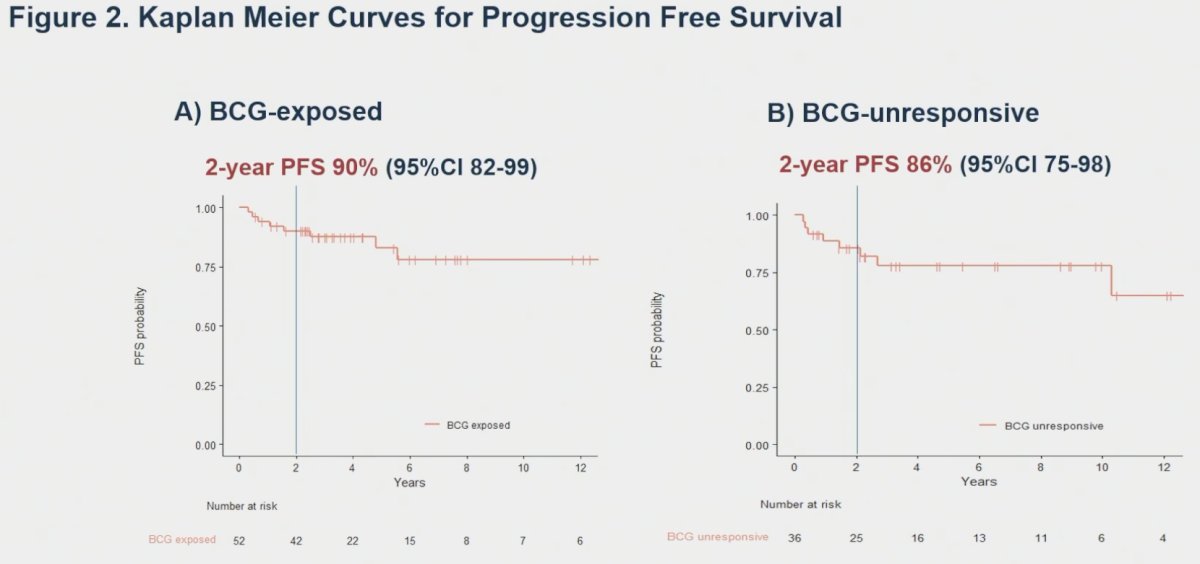
Dr. Myers noted that there are important limitations to this study:
- These results reflect those of selected patients receiving care at a single high-volume center
- They were unable to discern any association of stage or presence of CIS with outcomes
- Patients with lymphovascular invasion, variant histology, or hydronephrosis were not considered for additional BCG in this series
Dr. Myers concluded by noting that these results provide reference data for response rates to additional BCG, acting as a reference comparator for non-randomized studies and informing power calculations for randomized studies. Given the high response rates in the BCG-exposed group, it is essential that clinical trials in this population compare against BCG as a control arm.
Presented by: Amanda Myers, MD, Society of Urologic Oncology Fellow, Department of Surgery, The University of Texas, MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024


