(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured the AUA-IBCG Bladder Cancer Forum and a series of presentations by Drs. Kamal Pohar, Yair Lotan, and Kelly Bree discussing the role of urinary markers in surveillance of NMIBC patients. As moderator, Dr. Lotan started with a case presentation of a 72 white male with gross hematuria, and medical history of hypertension, hyperlipidemia, and a 20-pack-year smoking history. His CT urogram showed that the upper tracts were normal, there was no adenopathy, and the bladder was normal. The office cystoscopy showed two papillary tumors at the right lateral wall, 1 cm and 6 mm in diameter:
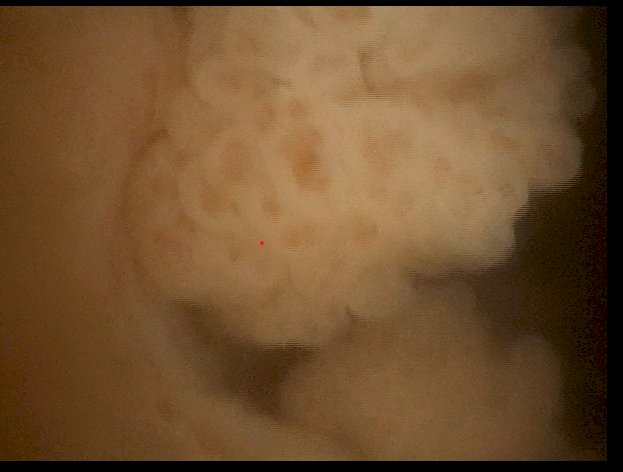
TURBT was performed with a post-operative dose of gemcitabine, pathology showing a low grade Ta tumor and no lamina propria invasion. Surveillance cystoscopy at 3 months was negative, as was urine cytology. Dr. Lotan then polled the audience with the following question “Do you use urine markers (other than cytology) to reduce cystoscopy intervals?”
- Yes: 15%
- No: 85%
With intermediate risk disease, what is the plan for surveillance? What is the interval for cystoscopy and should we use cytology? Are there other urine markers we can use? Dr. Bree then took the pro side of using urinary markers in the surveillance of non muscle invasive bladder cancer patients. Cystoscopy remains the gold standard for the diagnosis of bladder cancer, both at initial diagnosis and during surveillance, but there are limitations. Furthermore, it is costly (high risk non muscle invasive bladder cancer costs the US $373 million annually), there is pain/anxiety associated with the procedure, and the sensitivity is < 100%. Importantly, Dr. Bree notes that the AUA risk stratification for intermediate risk disease includes high grade Ta <= 3cm, however, many experts feel that any high grade lesions should be in the high risk category:
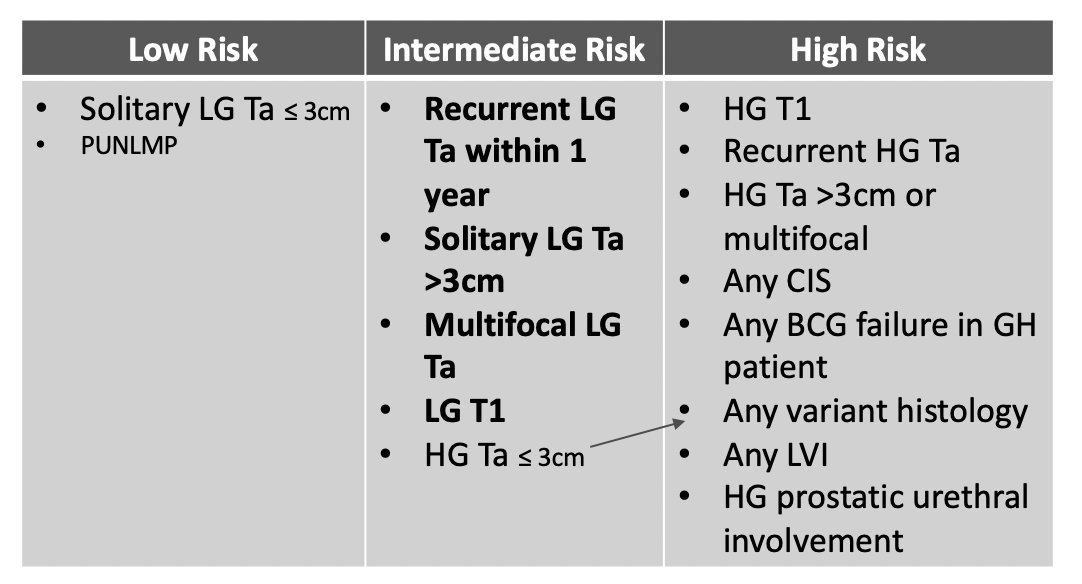
For patients with low grade Ta tumors, they are at high risk of recurrence, but cancer specific mortality is <1%, and 15 year progression free survival is >95%. Based on the guideline recommended follow-up for intermediate risk disease, a patient would undergo 8-14 cystoscopies in the 5 years, thus utilization of a marker could allow for fewer cystoscopies with minimal risk of poor oncologic outcomes. Dr. Bree notes that most biomarkers have poor sensitivity for low grade tumors, however, detection of high grade tumors is what is most important:

Dr. Bree’s proposed surveillance plan would include a urinary marker +/- cytology every 6 months, delaying cystoscopy to every 1-2 years, which could result in >10 fewer cystoscopies over a 5 year period of time.
Dr. Pohar then took the stance that no, we should not be using urinary markers in the surveillance of non muscle invasive bladder cancer patients. For intermediate risk disease, if the practical goal is to reduce the number of cystoscopies, he notes that based on the NCCN bladder cancer guidelines relative to low risk disease, only 2 additional cystoscopies are recommended within the first 5 years (a 6 and 18-month cystoscopy):
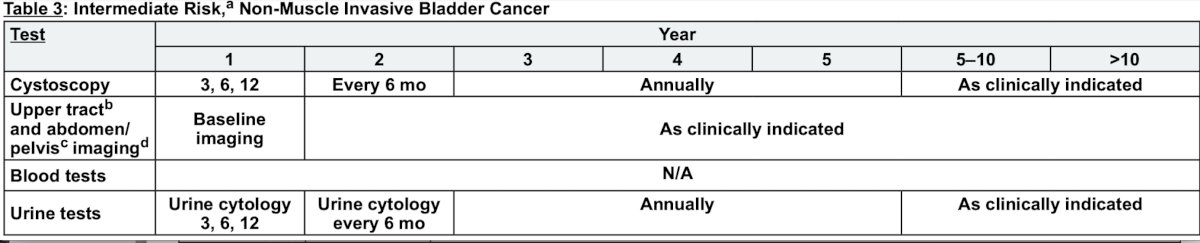
According to the AUA/SUO non-muscle invasive bladder cancer guidelines (2016, 2020, 2024), single protein and cell based biomarkers have a sensitivity in surveillance of 60-70%, but specificity is lower than cytology (70-80%). More recent multiplex assays for protein/mRNA/DNA methylation suggest higher sensitivity, but specificity remains similar to other urinary biomarkers. Importantly, low grade tumors have limited number of patients included in the studies, and overall sensitivity is no better than cytology.
In 2021, Reyes and colleagues1 did a randomized feasibility trial comparing surveillance regimens for patients with low and low-intermediate risk non-muscle invasive bladder cancer. The arms were high frequency cystoscopy (q3 months x 2 years, q6 months x 2 years, and then annually) versus low frequency cystoscopy (3 months, 9 months, annually), and of the 70 patients approached, 25 (36%) refused to be randomized to the low frequency group. This strongly suggests that patients desire knowledge of tumor recurrence in a timely manner. As such, the greatest limitation of current studies is study design, and what we need is multi-center, prospective randomized studies that are based on a trial design of a clinical cystoscopy algorithm that uses biomarkers in a decision tree analysis.
Dr. Lotan then discussed a second case of an 81-year-old white male with a medical history of hypertension and hyperlipidemia who presented with dysuria and microscopic hematuria. High grade Ta + CIS bladder cancer was diagnosed and he was started on 6 weeks of induction BCG. In follow-up, his 3-month cystoscopy and cytology were negative. Dr. Lotan then polled the audience with the following question “Do you use urine markers to adjudicate atypical cystoscopy or cytology?”
- Yes: 45%
- No: 55%
Dr. Bree, arguing for biomarkers in this scenario, noted that cystoscopy for surveillance is costly, and it is common to have significant inflammation after BCG therapy. Moreover, equivocal urine cytology is common (>20% of patients), and post-BCG inflammation can affect the accuracy of urine cytology. Thus, urine biomarkers can aid in the identification of recurrence and prevent unnecessary procedures. In the AUA guidelines, statement 11 states “In a patient with non-muscle invasive bladder cancer, a clinician may use biomarkers to assess response to intravesical BCG and adjudicate equivocal cytology (Expert Opinion).”
Many novel biomarkers show superior sensitivity and NPV compared to cytology and negative biomarker tests could aid in the prevention of unnecessary cystoscopies/TURBTs. According to Dr. Bree, while there is likely a role for markers with atypical cytology and bladder wall erythema, there is unlikely a role for biomarkers in the situation of a negative cystoscopy and negative cytology. In this scenario, the addition of a biomarker is unlikely to improve tumor detection but will add cost given that an “anticipatory positive” can occur in ~30% of patients.
Dr. Pohar then discussed the lack of a role of biomarkers in this setting, by emphasizing the AUA/SUO guideline statement 9 “In surveillance of non-muscle invasive bladder cancer, a clinician should not use a urinary biomarker in place of cystoscopic evaluation (Strong Recommendation; Evidence Strength: Grade B). In the setting of high risk non muscle invasive bladder cancer, the practical goal of urinary biomarkers is to predict durable response to BCG and adjudicate abnormal cystoscopy and/or cytology. Dr. Pohar notes that an anticipatory positive urinary biomarker may be secondary to residual disease (a true positive), early carcinogenesis/epithelial field effect, or it is a false positive.
Dr. Lotan was the lead author on a study evaluating the fluorescence in situ hybridization test to predict recurrence and/or progression of disease after BCG.2 Among 150 patients in this study, at 9 months of follow-up there were 46 events, including 37 recurrences and 9 progressions. For events with positive fluorescence in situ hybridization findings, the HR was 2.59 (95% CI 1.42-4.73) for the baseline test, 1.94 (95% CI 1.04-3.59) for the 6-week test, and 3.22 (95% CI 1.65-6.27) at 3 months:
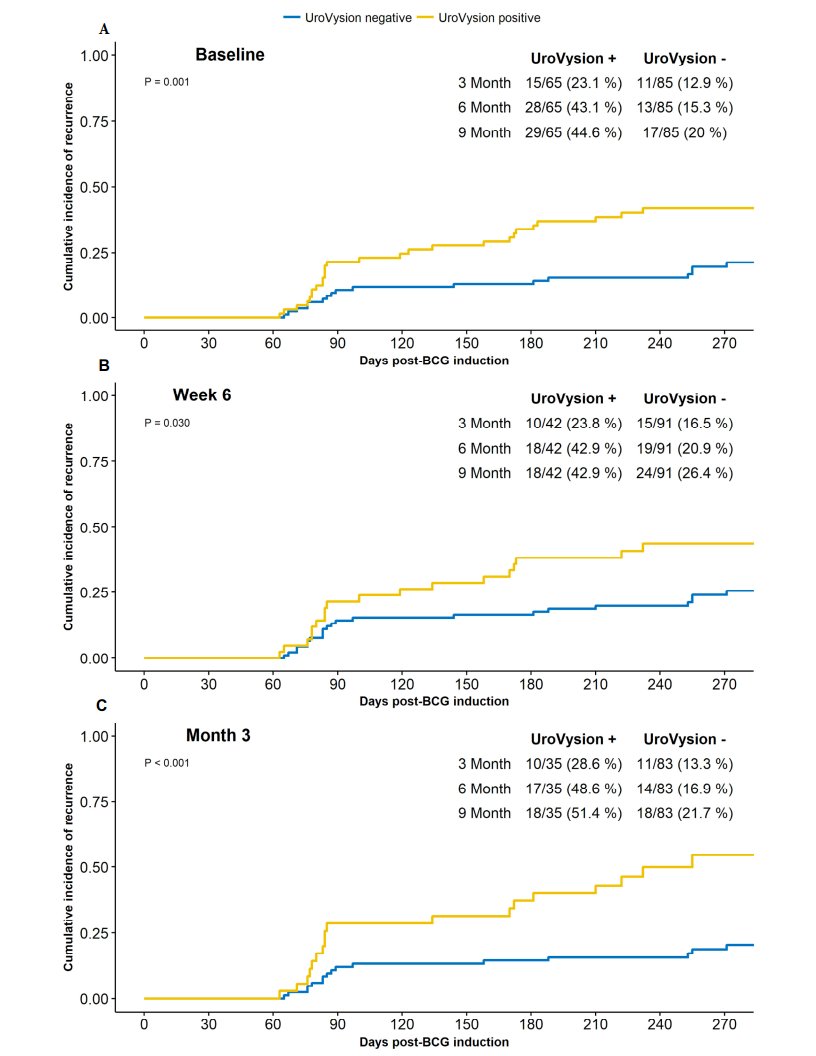
Thus, a positive test was associated with a 3.3 fold increased risk of recurrence, but sensitivity (36%), specificity (76%), PPV (40%), and NPV (73%) were all relatively poor. Dr. Pohar notes that in this study there was discordance between the results of the urinary biomarker and individual patient outcome (ie. a low PPV), with variability in the test result at three time points studied in a given patient, including 31% of patients with later recurrence events.
For adjudicating an abnormal cystoscopy and/or cytology, Dr. Pohar emphasized that we’ll always have The Paris System for Reporting Urinary Cytology, which released version 2 in 2022 after continued refinement. The major goal of version 2 was decreasing reporting of atypical urothelial cells, which is now down to 5-15%. With regards to blue light cystoscopy, Dr. Pohar states that enhanced cystoscopy is the best biomarker for adjudicating abnormal cystoscopic findings and that an emphasis should be placed on technological improvements.
Dr. Lotan closed this portion of the session by making the following summary statements:
- Intermediate risk low grade disease
- The risk of progression is low
- Alternating markers with cystoscopy is likely safe
- Evidence needs to allow inclusion into the guidelines
- Negative cystoscopy and cytology
- No current role
- We need data to support the utility of prognostic information
- Atypical cytology and/or cystoscopy
- A marker can inform which patients to biopsy
- Atypical cytology is less common due to the new PARIS classification system, but red patches abound
Moderator: Yair Lotan, MD, UT Southwestern, Dallas TX
Debaters:- Kamal Pohar, MD, Ohio State University, Columbus, OH
- Kelly Bree, MD, MD Anderson Cancer Center, Houston, TX
References:
- Reyes Rm, Rios E, Barney S, et al. A randomized feasibility trial comparing surveillance regimens for patients with low and low-intermediate risk non-muscle invasive bladder cancer. Bladder Cancer. 2021;7(3):285-295.
- Lotan Y, Inman BA, Davis LG, et al. Evaluation of the Fluorescence In Situ Hybridization Test to Predict Recurrence and/or Progression of Disease after bacillus Calmette-Guerin for Primary High Grade Nonmuscle Invasive Bladder Cancer: Results from a Prospective Multicenter Trial. J Urol. 2019 Nov;202(5):920-926.


