(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX was host to the Society of Urologic Oncology (SUO) session Dr. Jonathan Rosenberg discussed how to incorporate Enfortumab with or without Pembrolizumab into clinical practice.
Dr Rosenberg began his presentation by saying that Enfortumab vedotin (EV) and pembrolizumab have transformed the care of locally advanced and metastatic urothelial carcinoma. For the first time since platinum-based chemotherapy became the standard of care for this space of the disease, a new combination has been able to displace it as front-line therapy for advanced disease. To date, neoadjuvant chemotherapy continues to be cisplatin-based for eligible patients with muscle invasive urothelial carcinoma. However, clinical trials in the neoadjuvant setting are ongoing, but there is only reported data on EV monotherapy. The role of consolidative surgery after EV + Pembrolizumab could be considered in selected patients, especially in those with complete response when the evidence becomes available.
Dr. Rosenberg went on to discuss the mechanism of action of EV, briefly this is an antibody-drug conjugate (ADC) comprised of a human IgG1 antibody directed against Nectin-4 linked to monomethyl auristatin E (MMAE), a microtubule-disrupting agent. Nectin-4 is highly expressed in urothelial cancers. The internalization of the ADC-Nectin-4 complex, and the release of MMAE induces cell cycle arrest and apoptotic cancer cell death. (Figure 1)
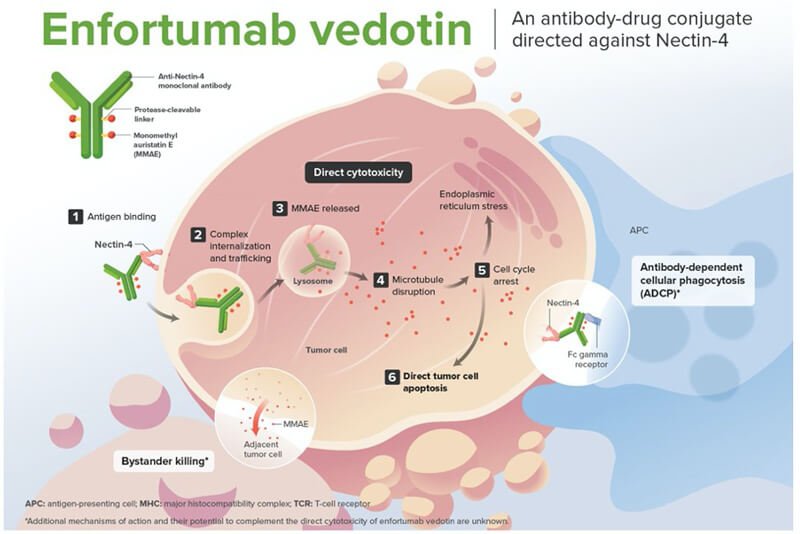
He further discussed the EV-301 trial, of which he was a co-author. This phase 3 trial evaluated EV for treating patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-based chemotherapy and experienced disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. The trial demonstrated that EV improved overall survival compared to standard chemotherapy.1
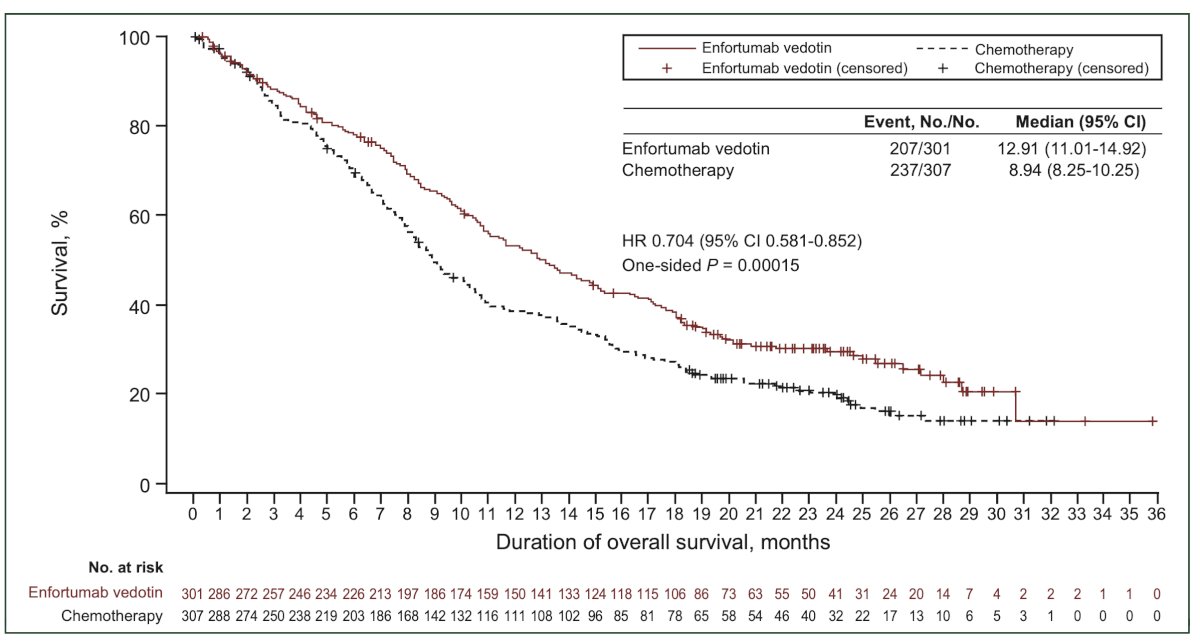
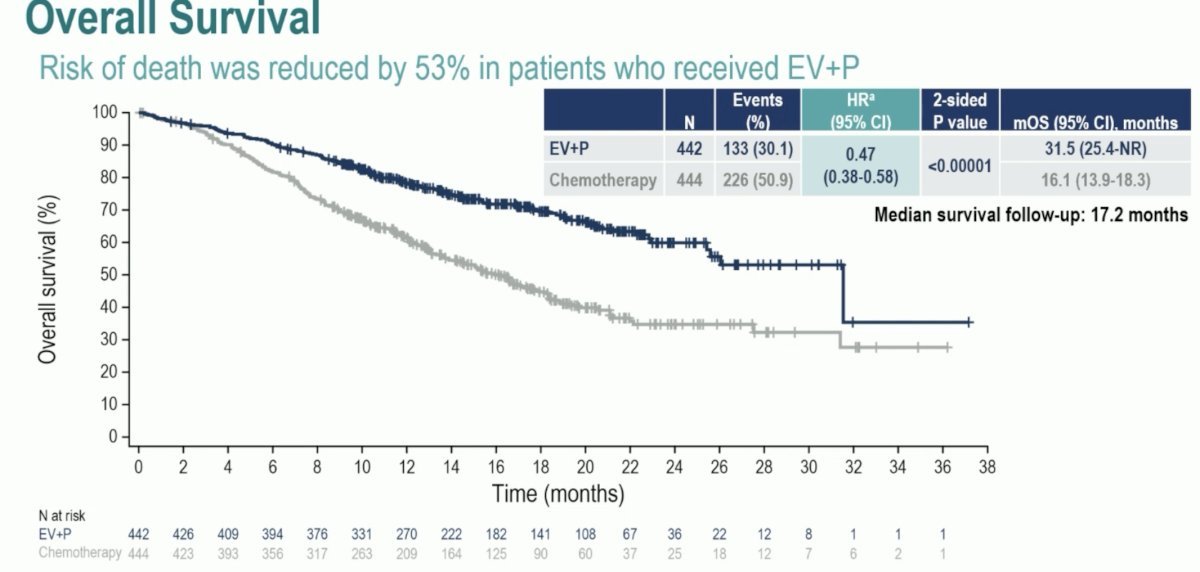
More recently, the EV-302/KEYNOTE-A39 trial was presented at ESMO 2023. This was an open label, randomized, controlled Phase 3 study evaluating EV in combination with pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma. The study enrolled 886 patients eligible for cisplatin- or carboplatin-containing chemotherapy regardless of PD-L1 status. Patients were randomized to receive either EV in combination with pembrolizumab or chemotherapy. The EV-302 trial demonstrated a significant reduction in the risk of progression/death (HR 0.45; 95% CI 0.38-0.54, p<0.00001) and overall survival (HR 0.47; 95% CI 0.38-0.58, p<0.0001) in patients treated with the combination EV + Pembrolizumab. Below is the Kaplan-Meier overall survival graphic.
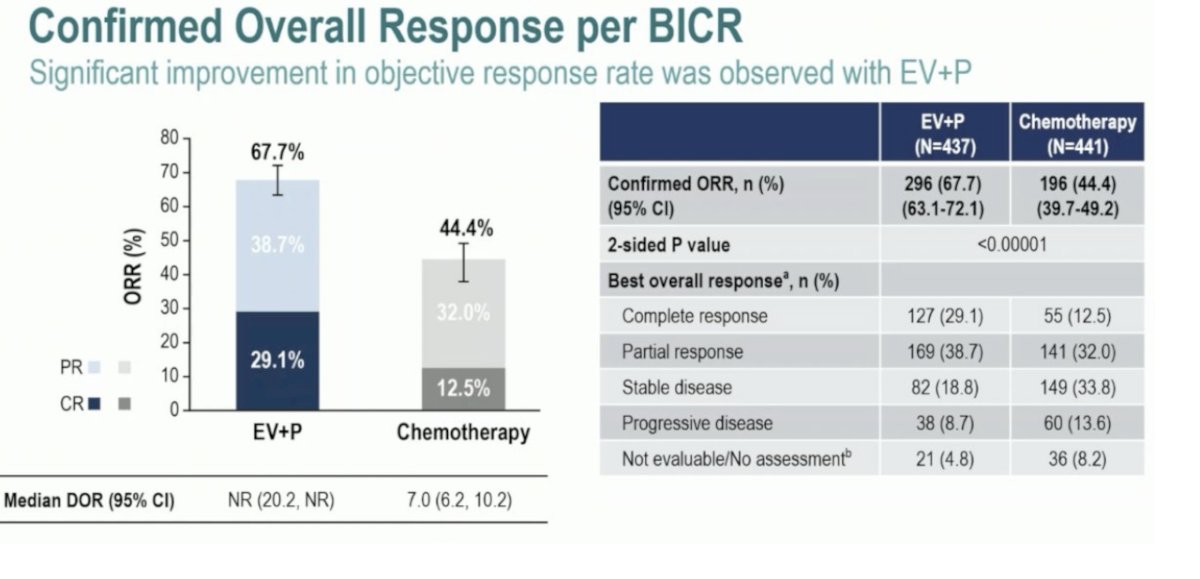
Additionally, the EV+Pembrolizumab combination yielded an impressive overall response of 67.7% vs 44.4% in the chemotherapy arm. In the combination arm, 29.2% of the patients had a complete response compared to 12.5% in the chemotherapy arm.
Dr. Rosenberg emphasized that EV toxicity is not negligible and can occur rapidly, occasionally with severe manifestations. He noted that early severe skin toxicity may necessitate therapy discontinuation. Additionally, severe refractory hyperglycemia should be screened and recognized early. Early recognition of toxicity and appropriate interventions, such as delaying doses, dose reduction, or discontinuation of therapy, are required when using EV. Generally, long-term toxicity manifests as peripheral neuropathy, typically dose-dependent and slow to resolve.
Moreover, Dr. Rosenberg presented the EV-103 cohort H study, which evaluates neoadjuvant EV for three cycles in cisplatin-ineligible patients with cT2-T4aN0M0 disease confined to the bladder and >50% urothelial carcinoma. To date, 22 patients have been enrolled, with an event-free survival rate of 76.4% at 12 months of follow-up. The pathological complete response (ypT0N0) rate was 36.4%.
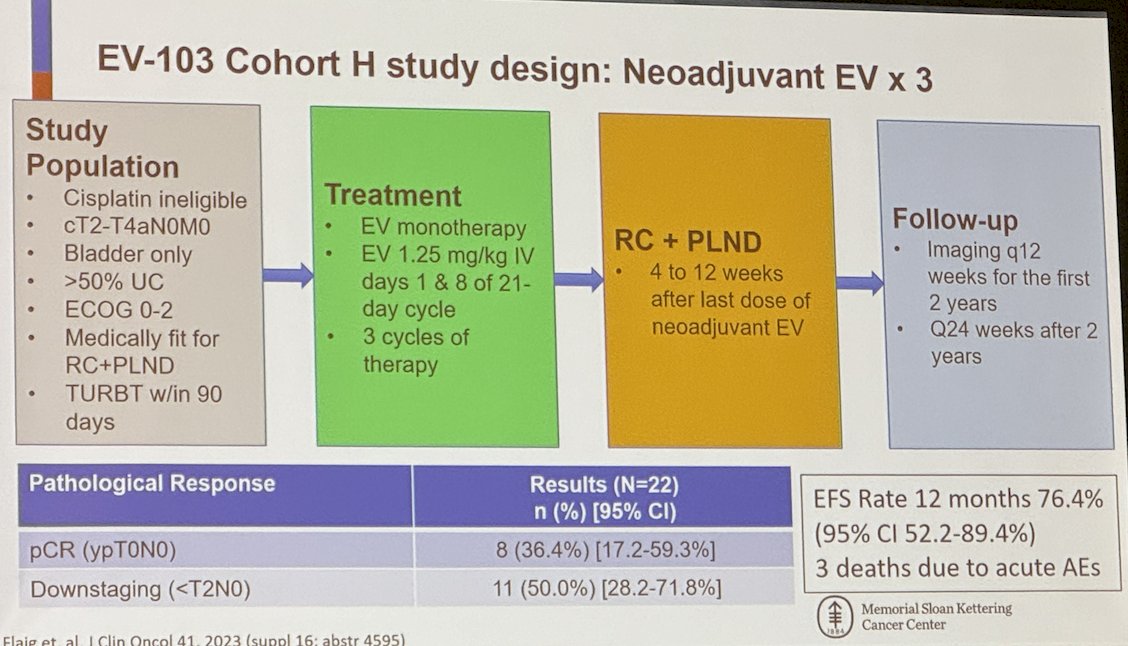
The EV-301 Cohort L uses a study design similar to that of Cohort H; however, the patients included in this trial also received 6 cycles of adjuvant EV after surgery. In this cohort, 50 patients have been enrolled, and 82.4% have completed 3 cycles of EV and surgery. The pCR rate was 34%.
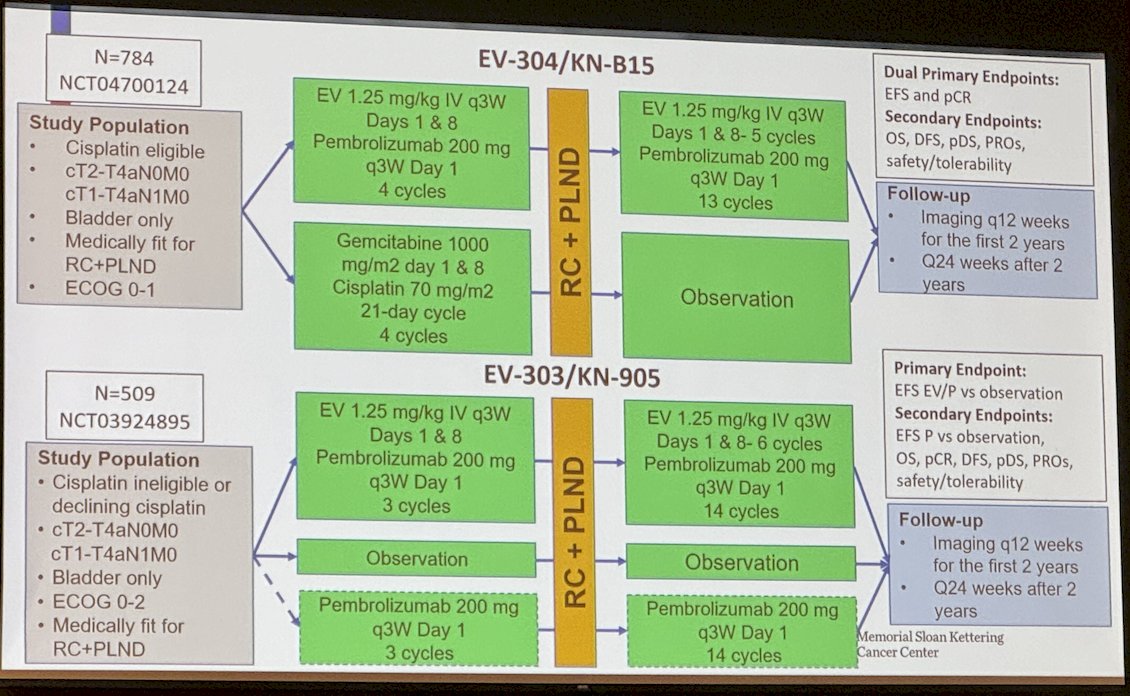
Dr. Rosenberg discussed two ongoing trials studying EV. The EV 304/KNB15 (NCT04700124) trial is studying cisplatin-eligible patients with cT2-T4aN0M0 or cT1-T4aN1M0 bladder cancer fit for radical cystectomy and pelvic lymph node dissection. It randomizes them to EV + Pembrolizumab for four cycles or Gemcitabine cisplatin followed by cystectomy and adjuvant EV + Pembrolizumab in the treatment arm for 13 cycles. Finally, he discussed the EV-303/KN-905 trial (NCT03924895) that includes cisplatin-ineligible patients or those declining cisplatin with cT2-T4aN0M0 or cT1-T4aN1M0 bladder cancer and randomizes them into three arms: i) Neoadjuvant EV + pembrolizumab for 3 cycles followed by adjuvant EV + Pembrolizumab for 14 cycles, ii) Observation, and iii) Pembrolizumab for 3 cycles followed by adjuvant pembrolizumab for 14 cycles.
Dr. Rosenberg concluded his presentation by saying:
- EV + pembrolizumab is transforming urothelial cancer care.
- Toxicity can be significant and may prevent curative therapy in a small subset of muscle invasive urothelial carcinoma patients.
- Management of toxicity is important to ensure optimal outcomes.
- Consolidative surgery should be considered for excellent responders with stage IV disease, similar to current practice.
- Optimal duration of therapy prior to consolidation is unclear.
- Based on magnitude of benefit of EV + Pembrolizumab in advanced UC, positive phase III trials in perioperative setting are expected
Presented by: Jonathan Rosenberg, MD, Genitourinary Oncologist at Memorial Sloan Kettering Cancer Center
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024
References:
- Rosenberg JE, Powles T, Sonpavde GP, Loriot Y, Duran I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Mamtani R, Wu C, Matsangou M, Campbell M, Petrylak DP. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol. 2023 Nov;34(11):1047-1054. doi: 10.1016/j.annonc.2023.08.016. Epub 2023 Sep 9. PMID: 37678672.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.


