(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured the AUA-IBCG Bladder Cancer Forum and a series of presentations by Drs. Sarah Psutka, Robert Svatek, and Paramananthan Mariappan discuss the management of recurrent low-grade intermediate-risk bladder tumors. Moderator Dr. Robert Svatek started by presenting a case of a 68-year-old male with a history of hypertension, coronary artery disease, obesity, and previous tobacco use, who presented with gross hematuria. His office cystoscopy is as follows:
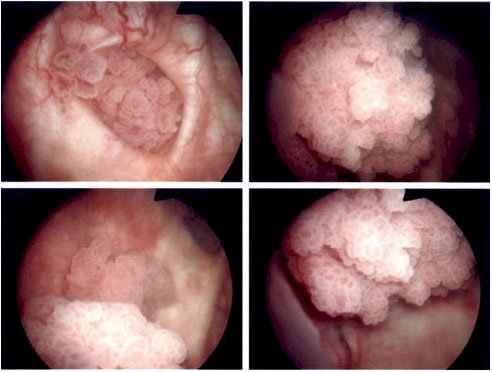
A subsequent TURBT demonstrated low-grade, multi-focal Ta urothelial carcinoma, AUA intermediate-risk disease. In the immediate postop period, the patient received intravesical gemcitabine, but should the patient receive adjuvant therapy? He subsequently was treated with six cycles of induction adjuvant intravesical BCG, followed by a 3-month cystoscopy that showed 2-3 small (<5 mm) papillary lesions scattered throughout the bladder, with a low-grade Ta appearance:
Dr. Psutka took the approach that it is time to de-escalate therapy and ablate or observe. The crux of the matter is: can we de-intensify therapy for recurrent low-grade urothelial carcinomas of the bladder? But, Dr. Psutka notes, before we get to that: why should we pursue treatment deintensification for patients with recurrent low-grade intermediate-risk nonmuscle invasive bladder cancer? Among 83,190 new bladder cancer diagnoses in 2024, ~61,000 will be nonmuscle invasive, and most low intermediate-risk tumors have a 5-year survival of 97%. Broadly speaking, treatment recommendations between and low and intermediate-risk diseases endorse a visually complete transurethral resection followed by a single dose of intravesical chemotherapy to reduce recurrence risk. For intermediate-risk disease, most guidelines endorse adjuvant therapy (BCG or chemotherapy) followed by 1 year of maintenance.
How long is cystoscopic surveillance necessary? In a multicenter retrospective observational study of 577 patients who received their first TURBT before 2016 for low-risk disease, Ma et al.1 found that recurrences are common, with up to 80% at 5 years, but are low grade in 95% of cases. The risk of progression to high-risk non-muscle-invasive bladder cancer is <3% and recurrent low-grade disease progressing to muscle-invasive bladder cancer in 5+ years is < 1%. Thus, these are not life-threatening recurrences.
There are major concerns regarding the current paradigm of the frequent resections and adjuvant therapy recommended for patients with recurrent low-grade non-muscle invasive bladder cancer. Importantly, if we take the patient's perspective, the frequent trips to the operating room are associated with a non-negligible risk of surgical complications. Among this predominantly older and comorbid patient population, there are also substantial risks associated with recurrent general anesthetics including a risk of up to 65% for postoperative delirium, as well as a 10% increased risk of long-term cognitive decline. Furthermore, from both the patient's perspective, and a societal perspective, we must consider the costs to patients and the health care system of not only the frequent surveillance but also recurrent TURBTs. The cost of intermediate-risk nonmuscle invasive bladder cancer is estimated over 5 years to be ~$150,000 per patient.
Key point #1 according to Dr. Psutka is: We have the rationale to pursue de-intensification of treatment for frequent low-grade recurrences of low-grade intermediate-risk nonmuscle invasive bladder cancer. The next important question is: What are the requirements to accept treatment deintensification as the new standard?
- Improved (or similar) efficacy
- Reduced harms
- Satisfactory to patients
- Negligible impact on the future treatment eligibility and efficacy
If we do not re-resect/retreat, how can we handle low-grade intermediate-risk nonmuscle invasive bladder cancer recurrences? Three strategies are: active surveillance, office fulguration, or chemoablation. Dr. Psutka notes that active surveillance is a topic that urologists are very comfortable with and implement across multiple other disease scenarios. This was first popularized by Dr. Mark Soloway and colleagues who demonstrated the feasibility of active surveillance in low-grade disease, highlighting the reliability of surgeons experienced at identifying recurrent low-grade tumors on flexible office cystoscopy.
The Bladder Cancer Italian Active Surveillance (BIAS) protocol subsequently reported on 214 patients on active surveillance for a median of 38.8 months.2-3 Inclusion criteria was a history of low-grade pTa/pT1a urothelial carcinoma, and < 5 tumors < 1cm in size. Triggers for treatment were an increase in size/number, gross hematuria, or positive cytology. This study found that 49% of patients never had to undergo TURBT, while 44% remained on active surveillance > 18 months. Treatment-free survival is as follows:
Among those who underwent TURBT, 19% did not have cancer and 71% had recurrent low-grade pTa. Importantly, this led to reduced resource consumption: $1,360-$1,500/TURBT and 1.9 hospital beds avoided.
A 2024 AUA Update series highlighted the current literature and suggests that patients like active surveillance (compliance is excellent), it is feasible (median time on active surveillance: 9-95 months, re-TURBT was avoided in up to 70% of cases), and it was effective/safe (grade progression < 20%, stage progression < 13% and progression to muscle-invasive bladder cancer was only seen in high-grade pT1):
Ultimately, patient selection is key and this should be used for only low-grade recurrence. The use of cytology helps to rule out a high-grade recurrence.
Office fulguration is also feasible, effective, and patients like it. There is a low risk of reclassification to high-risk nonmuscle invasive bladder cancer (~5-20%), the accuracy of predicting low-grade disease is 85-93%, for 1 cm tumors, the 2-year recurrence-free survival is similar to TURBT (28% vs 26%), VAS of 0-3 (no, mild pain) is noted in 86% of patients, with 97% of patients preferring office fulguration under local anesthesia rather than going to the operating room.
With regards to chemoablation, this is essentially a de-escalation strategy to avoid unnecessary trips to the operating room and general anesthesia. Based on prior in vitro investigations demonstrating that urothelial cancer cell lines duplicate within less than 3 days, some investigators have hypothesized that an increased tumor-killing effect may be observed by administering intravesical chemotherapy on a more intensive schedule (every 48-72 hours). In a nonrandomized observational study by Rabioppi et al.,4 outcomes were compared between patients undergoing an intensive, three-times weekly mitomycin C instillation for 2 weeks to a standard protocol of TURBT followed by weekly induction mitomycin C. There were similar complete response rates between the two study groups:
Additionally, there was avoidance of a TURBT in >70% of cases following recurrence of low/intermediate risk nonmuscle invasive bladder cancer, and no difference in cancer-free survival (median follow-up of 39 months). Toxicity was also low: there was no systemic toxicity, no patients discontinued therapy and local toxicity was similar to TURBT: 21.3% vs 27.6% (p = 0.32).
In the ATLAS trial,5 the 3-month complete response rate for UGN-102 was 65% compared to 64% for TURBT, and the 15-month disease-free survival for UGN-102 was 72% compared to 50% for TURBT. Dr. Prasad presented the results of the ENVISION trial at SUO 2023, which is a phase 3, single-arm, multi-center study to evaluate the efficacy and safety of UGN-102 as primary chemoablative therapy in patients with low-grade nonmuscle invasive bladder cancer at intermediate risk of recurrence (n = 240), reporting a 3-month complete response rate of 79.2% (14% residual disease, 2.5% progression to high-grade disease). In a systematic review of chemoablative studies published in 2024 by Yanagisawa et al.,6 they examined 23 studies including 1,199 patients undergoing intravesical chemotherapy (4-8 weekly treatments of standard chemotherapy or chemo hyperthermia). The pooled complete response rates were 50.9% (95% CI 45.9-55.9%) for the marker lesion and 48% (95% CI 56.2-72.3%) for small tumors. Based on these results, Dr. Psutka summarizes that we can potentially avoid TURBT in approximately half of the patients with recurrent small low-grade urothelial carcinoma. Moreover, intensive mitomycin C regimens are associated with less dysuria and frequency than TURBT + adjuvant mitomycin C.
Another strategy to consider for leveraging our knowledge of tumor biology of intermediate-risk nonmuscle invasive bladder cancer is to target therapy with the goal of improving treatment efficacy. DNA damage response gene mutations in resected tumors are a potential biomarker to select those patients who would most benefit from novel drug delivery methods. One critical recent observation regarding these tumors is that low-grade papillary tumors appear to arise via hyperplasia with minimal dysplasia. Additionally, these tumors are characterized at the molecular level by the loss of heterozygosity of chromosome 9 and activating mutations of FGFR3, which is expressed in approximately 60% of low-grade urothelial carcinomas. Erdafitinib is an FGFR1-4 inhibitor, and the TAR-210 trial cohort 3 will assess treatment efficacy in intermediate-risk nonmuscle invasive bladder cancer with a previous history of low-grade only Ta/T1 disease for those not planned for cystectomy.
Dr. Psutka’s key point #2 is that we have safe, effective options to achieve de-intensification of treatment for frequent low-grade recurrences of low-grade intermediate-risk nonmuscle invasive bladder cancer. To conclude, Dr. Psutka emphasized that in 2024, yes, recurrent low-grade papillary urothelial carcinoma can be managed without surgery in well-selected and well-counseled patients.
Dr. Mariappan then took the approach of TURBT + adjuvant treatment. Dr. Mariappan notes that there is intense molecular and clinical heterogeneity in nonmuscle invasive bladder cancer:7
The IBCG algorithm for intermediate-risk nonmuscle invasive bladder cancer stratifies patients by risk factor, and Dr. Mariappan emphasized that this patient has >= 3 risk factors and should be treated with TURBT + adjuvant induction and maintenance therapy:
In work led by Dr. Mariappan,8 they found that primary intermediate-risk nonmuscle invasive bladder cancer has a 5-year recurrence risk of 43% and 2% risk of progression, however, secondary intermediate-risk disease has a 5-year recurrence risk of 66% (vs primary: OR 2.5, 95% CI 1.6-3.8) and a 15% progression risk: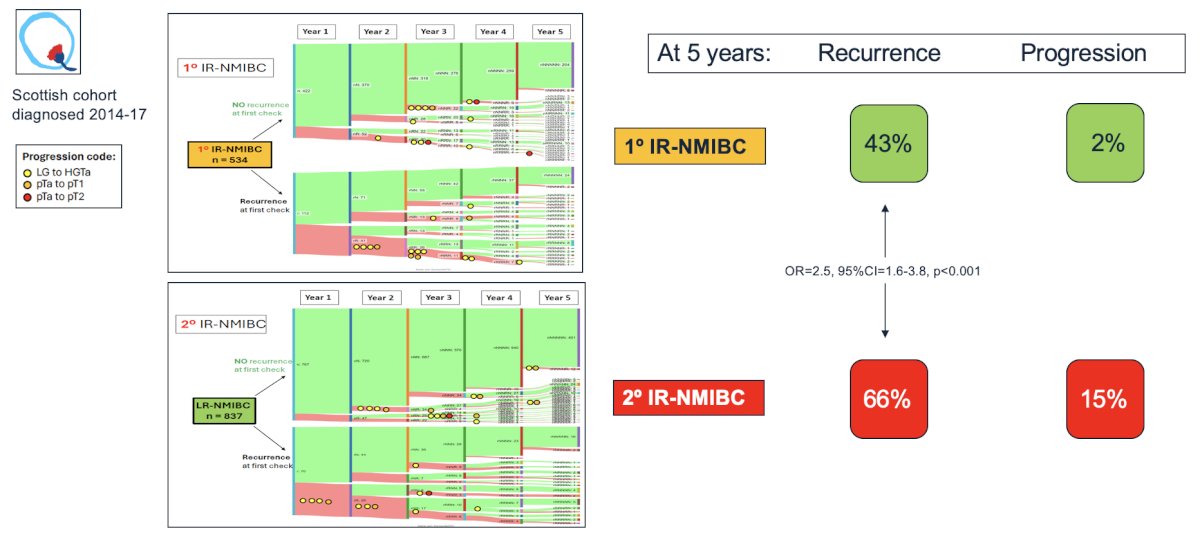
Dr. Mariappan emphasized that clear diagnostics and documentation of tumor characteristics are vital to an effective risk-adapted approach in nonmuscle invasive bladder cancer. Moreover, apart from a field change, there is intra-epithelial spread and luminal seeding. Thus, visual evaluation is also flawed. Dr. Marriapan then discussed a systematic review assessing the efficacy and safety of outpatient bladder tumor ablation which analyzed 1,584 patients from 17 studies. It is important to note that there were heterogeneous inclusion criteria and data, with low certainty evidence for any recommendations.9
Dr. Marriapan highlighted CALIBER, which was a UK phase II randomized feasibility trial of chemoablation with mitomycin-C versus surgical management in low-risk non-muscle-invasive bladder cancer. What is important to note about this trial is that early recurrence with TURBT occurred in 12% of cases compared to 52% of patients undergoing chemoablation: 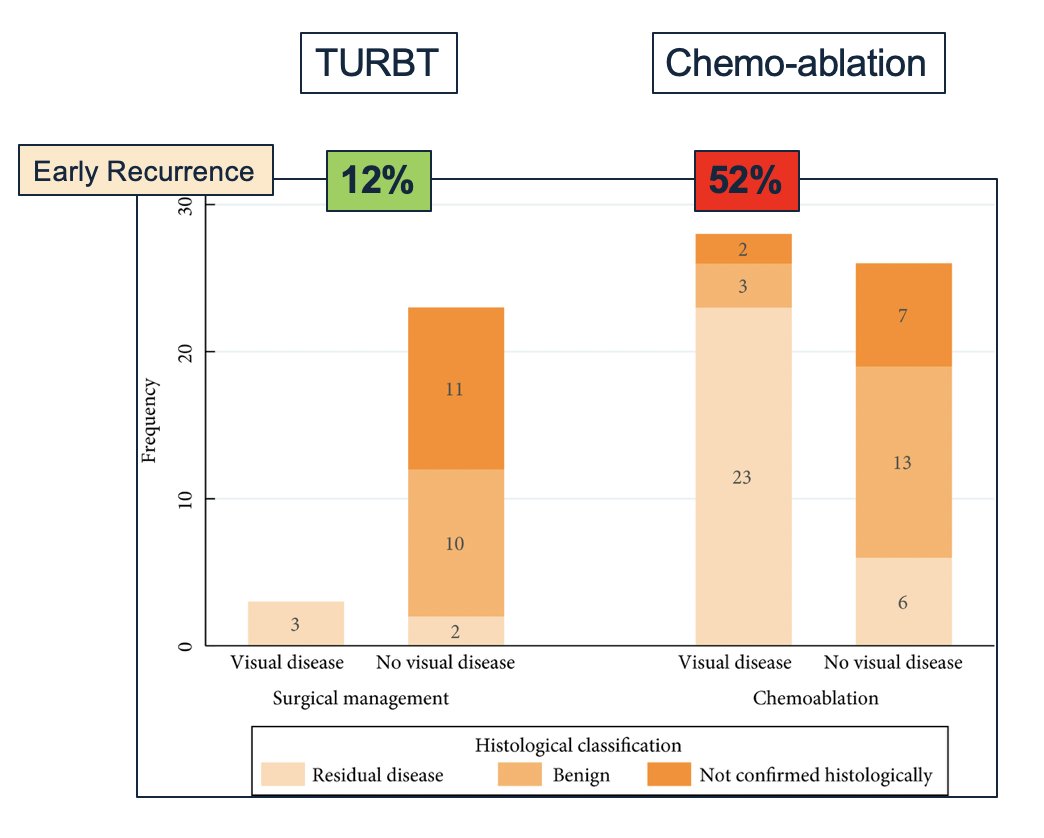
Dr. Marriapan notes that active surveillance is an onerous and laborious endeavor, with frequent cystoscopy, frequent cytology, and in need of an experienced cystoscopist:
An additional concern is an ability to discern a high versus a low-grade tumor, with 20% of tumors deemed low-grade actually high-grade. Quality of life is also an important consideration for these patients, with data from CALIBER suggesting those that underwent chemoablation had a 3 and 6-month worse quality of life compared to TURBT, which essentially resolved at 12 months:
Dr. Mariappan concluded his portion of the debate by using the acronym IR-NMIBC to make his closing remarks:
IBCG leveraged data provides a risk-adapted algorithm
Risk-adapted protocol
-
Nuanced approached
Mechanisms (all) of residual and recurrent disease are addressed
Intravesical adjuvant treatment (carefully)
Being mindful of impact on the patient’s quality of life
Counsel effectively and clearly
Moderator: Robert Svatek, MD, Surgeon Scientist, UT Health San Antonio, San Antonio, TX
Debaters:
Sarah Psutka, MD, MS, Urologic Oncologist, Associate Professor of Urology, Department of Urology, Fred Hutchinson Cancer Research Center, University of Washington, Seattle, WA
Paramananthan Mariappan, Professor, Urological Surgeon, Director Edinburgh Bladder Cancer Surgery, Edinburgh Bladder Cancer Surgery, Edinburgh, Scotland
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 - Mon, May 6, 2024.
Related content: To TURBT or Not to TURBT? Evaluating Treatment Options for Recurrent Low-Grade, Intermediate-Risk Bladder Tumors - Sarah Psutka & Param Mariappan
References:
- Ma J, Roumiguie M, Hayashi T, et al. Long-term recurrence rates of low-risk non-muscle-invasive bladder cancer: How long is cystoscopic surveillance necessary? Eur Urol Focus. 2024 Jan;10(1):189-196.
- Hurle R, Lazzeri M, Vanni E, et al. Active surveillance for low risk nonmuscle invasive bladder cancer: A confirmatory and resource consumption study from the BIAS project. J Urol. 2018 Feb;199(2):401-406.
- Contieri R, Paciotti M, Lughezzani G, et la. Long-term follow-up and factors associated with active surveillance failure for patients with non-muscle-invasive bladder cancer: The Bladder Cancer Italian Active Surveillance (BIAS) experience. Eur Urol Oncol. 2022 Apr;(5):251-255.
- Racioppi M, Di Gianfrancesco L, Ragonese M, et al. Chemoablation with intensive intravesical mitomycin C treatment: A new approach for non-muscle-invasive bladder cancer. Eur Urol Oncol. 2019 Sep;2(5):576-583.
- Prasad SM, Huang WC, Shore ND, et al. Treatment of low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102 +/- transurethral resection of bladder tumor compared to transurethral resection of bladder tumor monotherapy: A randomized, controlled, phase 3 trial (ATLAS). J Urol. 2023 Oct;210(4):619-629.
- Yanagisawa T, Kawada T, von Deimling M, et al. Repeat transurethral resection for non-muscle-invasive bladder cancer: An updated systematic review and meta-analysis in the contemporary era. Eur Urol Focus. 2024 Jan;10(1):41-56.
- Lindskrog SV, Prip F, Lamy P, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. 2021 Apr 16;12(1):2301.
- Mariappan P, Johnston A, Trail M, et al. Achieving benchmarks for National Quality Indicators Reduces Recurrence and Progression in Non-muscle-invasive Bladder Cancer. Eur Urol Oncol. 2024 Jan 30 [Epub ahead of print].
- Malde S, Grover S, Raj S, et al. A systematic review of the efficacy and safety of outpatient bladder tumor ablation. Eur Urol Focus. 2022 Jan;8(1):141-151.
- Mostafid AH, Porta N, Cresswell J, et al. CALIBER: A phase II randomized feasibility trial of chemoablation with mitomycin-C versus surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int. 2020 Jun;125(6):817-826.


