(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Bladder Cancer Non-invasive podium session. Dr. Matthew Clements presented the results of a longitudinal study assessing patient-reported toxicity at the Memorial Sloan Kettering Cancer Center (MSK).
Dr. Clements began his presentation by discussing that intravesical therapy remains the gold standard treatment for intermediate or high-risk non-muscle invasive bladder cancer (NMIBC) after transurethral resection of a bladder tumor (TURBT). However, up to 18% did not complete full induction of Bacillus Calmette-Guérin (BCG).
To date, BCG remains the most extensively studied therapy for this condition. However, despite over 40 years of utilization, the patient-reported toxicity of this treatment has not been distinctly characterized. Furthermore, while other intravesical therapies are commonly employed in clinical practice, they have yet to be systematically compared to the established gold standard, BCG. The objective of this study was to utilize a validated assessment tool to longitudinally compare the toxicity profiles associated with various intravesical therapies.
They included patients treated at MSK between June 2022 to September 2023. These patients were automatically sent a questionnaire through the electronic patient portal at the time when intravesical therapy was ordered, and three days after each scheduled intravesical instillation.
To measure the toxicity of therapy, the investigators used the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), and chose 18 relevant items from this questionnaire, to specifically evaluate constitutional, cardiopulmonary, gastrointestinal, mental, and urinary side effects of treatment. In this questionnaire toxicity grades range from 0-3.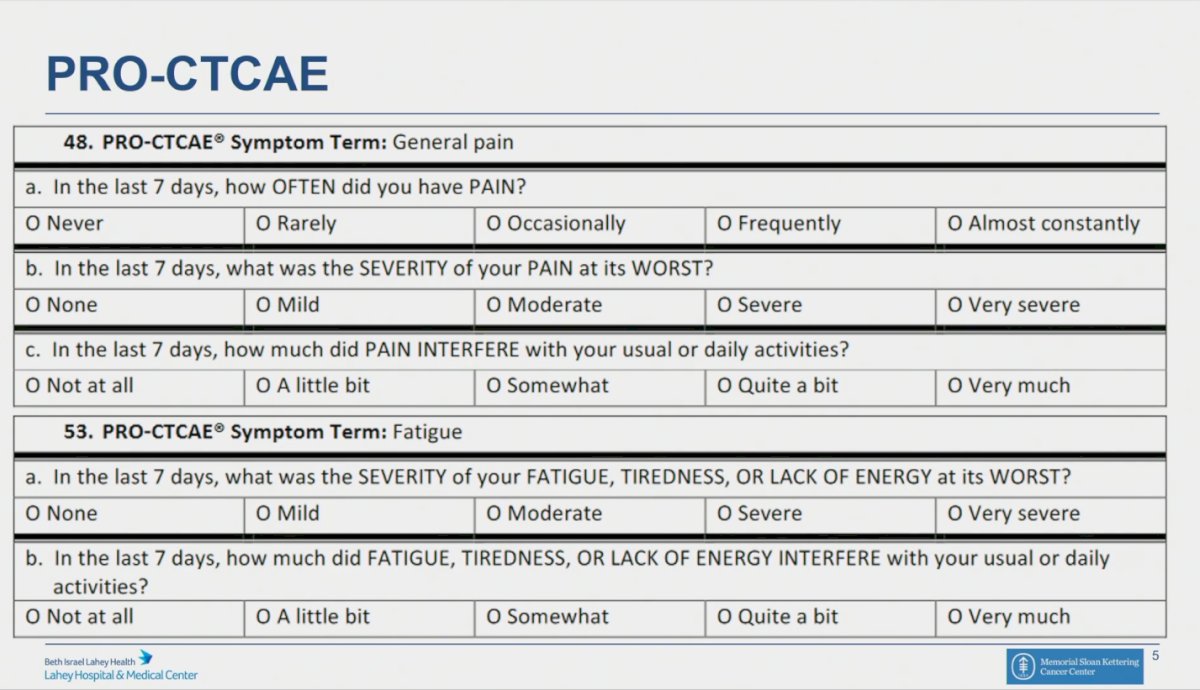
They use natural cubic splines fitted to mean scores to visualize outcomes and linear mixed effects models, adjusting for clinical factors, were used by the investigators to compare longitudinal toxicity.
The investigators included 267 subjects who completed at least one survey during therapy. Of these 199 received treatment with BCG, 35 with gemcitabine, and 29 with gemcitabine/docetaxel. Dr. Clements mentioned only 4 patients received mitomycin and therefore were not analyzed as a separate group.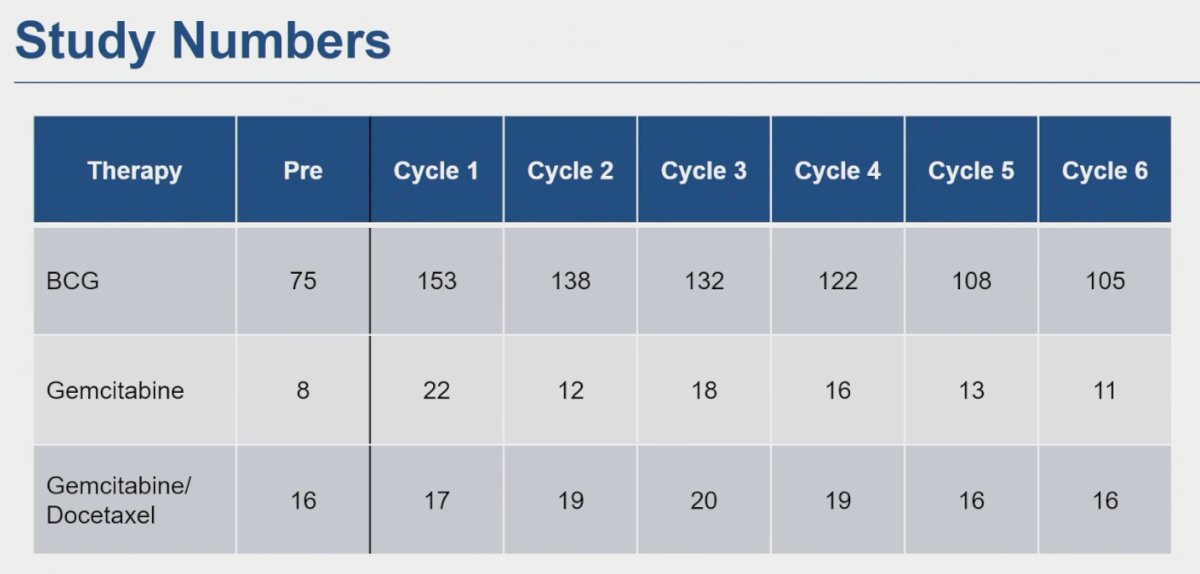
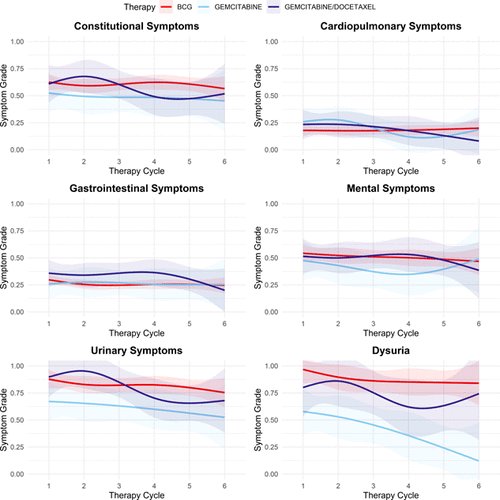
Each participant completed a median of 5 surveys. There was no discernible disparity in constitutional symptoms between BCG and gemcitabine (p=0.16) or gemcitabine/docetaxel (p=0.54) intravesical treatments. They found no significant difference in overall urinary symptoms compared to BCG (gemcitabine p=0.09, gemcitabine/docetaxel p=0.5). However, dysuria was more pronounced with BCG, reflected by a coefficient of -0.65 (p<0.001) for gemcitabine and -0.32 (p=0.048) for gemcitabine/docetaxel. Conversely, no significant differences were noted in gastrointestinal, mental, or cardiopulmonary symptoms. Overall, toxicity remained minimal, with mean toxicity grades in the PRO-CTCAE consistently below 1 (score 1-3) across all categories over the 6 weeks of intravesical instillations.
Dr. Clements finished his presentation by leaving the following conclusions:
- During the course of intravesical therapy, toxicity remained minimal for BCG, gemcitabine, and gemcitabine/docetaxel combinations, which was contrary to initial expectations.
- There was no indication of escalating toxicity observed throughout the treatment regimen.
- Further insights needed on maintenance therapy
- There is a dire need to compare this agent to novel intravesical therapies as well.
Presented by: Matthew Clements, MD, Urologic Oncologist, Lahey Institute of Urology, Burlington, MA
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024


