(UroToday.com) During the annual American Urological Association (AUA) meeting in San Antonio, Dr. Kimberly Maciolek and colleagues presented on artificial intelligent computer models for stone dust evaluation. The technique of dusting following laser ablation during flexible ureteroscopy (fURS) provides urologists with an efficient approach to stone management. However, it is estimated that 20-30% of patients will require surgical intervention within a two-year period1. Therefore, it can be surmised that endoscopic stone clearance can predict recurrence of stone disease2.
Despite its widespread use, there exists paucity of current data for objective metrics in the evaluation of stone dust, wherein the correlation between dust and stone-free status requires further investigation. At the Vanderbilt Institute of Surgery and Engineering, urologists have been able to develop computer vision models for stone segmentation during fURS and laser lithotripsy3. Such depictions merge artificial intelligence and machine learning to instruct computers for the processing of visual data akin to the mechanisms of the human brain. Accordingly, Dr. Maciolek and her team sought to employ artificial intelligent computer vision models based upon their prior work to assess stone dust following laser ablation and to predict stone-free status.
Following laser ablation with dusting settings, 70 videos of stone dust were collected in patients undergoing fURS for intrarenal stones. For further classification, these videos were identified based upon stone-free status observed on postoperative imaging (i.e., no residual fragments present). To determine postoperative stone-free status, Dr. Maciolek and her team established a deep convolutional neural network after extracting individual frames at 30 FPS. A previously validated stone segmentation model was included as an auxiliary input channel, while the output layer consisted of treatment outcomes including stone-free and residual stones present (Figure 1). During model training, labeled images and videos were provided, allowing the model to discern distinct patterns and features. In total, 80% of frames (n=26,592 from 54 videos) were utilized, while the additional 20% of frames (n=6,648 from 16 videos) were designated for testing to predict stone remnants, if any.

Figure 1: Computer Vision Model for Stone Dusting Assessment.
A threshold of 0.5 for model execution of frame classification was established, which was further assessed via accuracy, sensitivity, specificity, and area under the receiver operating curve (AUC-ROC). Moreover, the predictions derived from individual frames were consolidated to provide a prediction per video (i.e. per patient). There were no significant differences in either patient population (Figure 2). Of note, a majority of stones were calcium-based with similar volume and HU. Although these stones were present throughout the urinary tract, they were often relocated to the upper pole intraoperatively.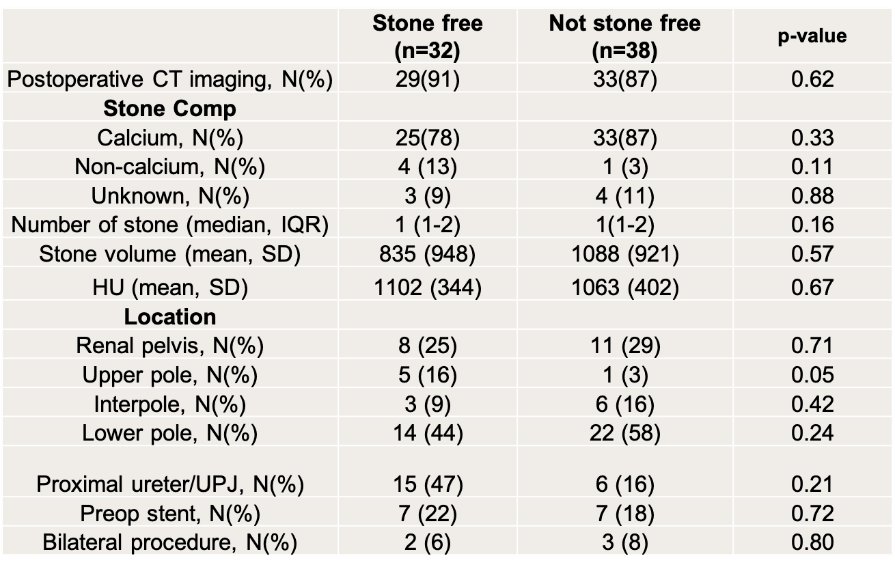
Figure 2. Comparison of Groups with Respect to Imaging and Stone Characteristics.
Postoperative CT imaging was performed in 61 patients (87%) and renal ultrasound in 9 patients (13%); on average, time to postoperative imaging was about 49 days (SD=20). Overall, 32 patients (46%) were stone-free, while 38 patients (54%) had residual stone fragments present on postoperative imaging. The average length of the video clips used for model training was 15 seconds (SD=7). With an accuracy of 0.70, sensitivity of 0.76, and specificity of 0.62, the model exhibited strong performance of frame classification by stone-free status. The AUC-ROC of the model was 0.76, among all four thresholds of frame classification. Indeed, there was higher accuracy classification per video achieved by the model (accuracy: 0.81). Furthermore, example videos of true positive stone-free (fine appearing dust) vs true negative residual stone (larger stone fragments) for model and postoperative imaging were demonstrated.
Notably, factors related to false positives and negatives remain undefined given the limitations of computer vision models. As Dr. Maciolek stated, “The model functions like a black box – we cannot provide an explanation of how the neural network reaches a conclusion. The model itself modifies the layer to optimize the output but can reach different conclusions based on the input.” Features that may interfere with such interpretation include edges, textures, patterns, or other objects based upon a pixel level. Furthermore, the limited quantity of videos and the exclusion of perioperative factors are additional study constraints.
In conclusion, Dr. Maciolek’s presentation exemplified the practical application of artificial intelligence with respect to the assessment of stone dust and further predicting stone-free rates. She wrapped up her podium presentation by imparting the following messages for the audience:
- These models demonstrated the possibility of prognosticating stone-free status based upon residual dust following stone ablation.
- Further applications and optimization of these models may enhance surgical efficiency and stone-free rates in real time.
- Future directions may include: compiling an extensive database, external model validation, open-source software, comparison to current predictive models, intraoperative decision-making, and clinical outcomes.
Presented by: Kimberly Maciolek, MD, Endourology Fellow, Vanderbilt University Medical Center, Nashville, TN
Written by: Mariah Hernandez, Department of Urology, University of California, Irvine, @mariahch00 on Twitter during the 2024 40th American Urological Association (AUA) Annual Meeting, May 3 — May 6, 2024, San Antonio, Texas
References:- Brain E, Geraghty RM, Lovegrove CE, Yang B, Somani BK. Natural History of Post-Treatment Kidney Stone Fragments: A Systematic Review and Meta-Analysis. J Urol. 2021 Sep;206(3):526-538.
- Hein S, Miernik A, Wilhelm K, Schlager D, Schoeb DS, Adams F, Vach W, Schoenthaler M. Endoscopically Determined Stone Clearance Predicts Disease Recurrence Within 5 Years After Retrograde Intrarenal Surgery. J Endourol. 2016 Jun;30(6):644-9.
- Setia SA, Stoebner ZA, Floyd C, Lu D, Oguz I, Kavoussi NL. Computer Vision Enabled Segmentation of Kidney Stones During Ureteroscopy and Laser Lithotripsy. J Endourol. 2023;37(4):495-501.


