(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a plenary session. Dr. Mohamad Allaf delivered a state-of-the-art lecture discussing advances in molecular imaging for renal tumors.
Molecular imaging is a branch of radiology that utilizes radiopharmaceuticals to diagnose and treat disease. It allows for the functional and/or molecular characterization of cellular processes. It is performed with either single photon emission computed tomography (SPECT) or positron emission tomography (PET) in combination with CT.

What are the differences between SPECT and PET? PET uses positron emitters (e.g., 18F, 68Ga), as opposed to photon emitters in SPECT, and has superior spatial resolution compared to SPECT. PET uptake is quantifiable (i.e., standardized uptake value [SUV]). However, PET is considerably more expensive than SPECT.

Can we leverage molecular imaging techniques to differentiate among the various renal tumor histologies? There are two goals here that have important treatment implications:
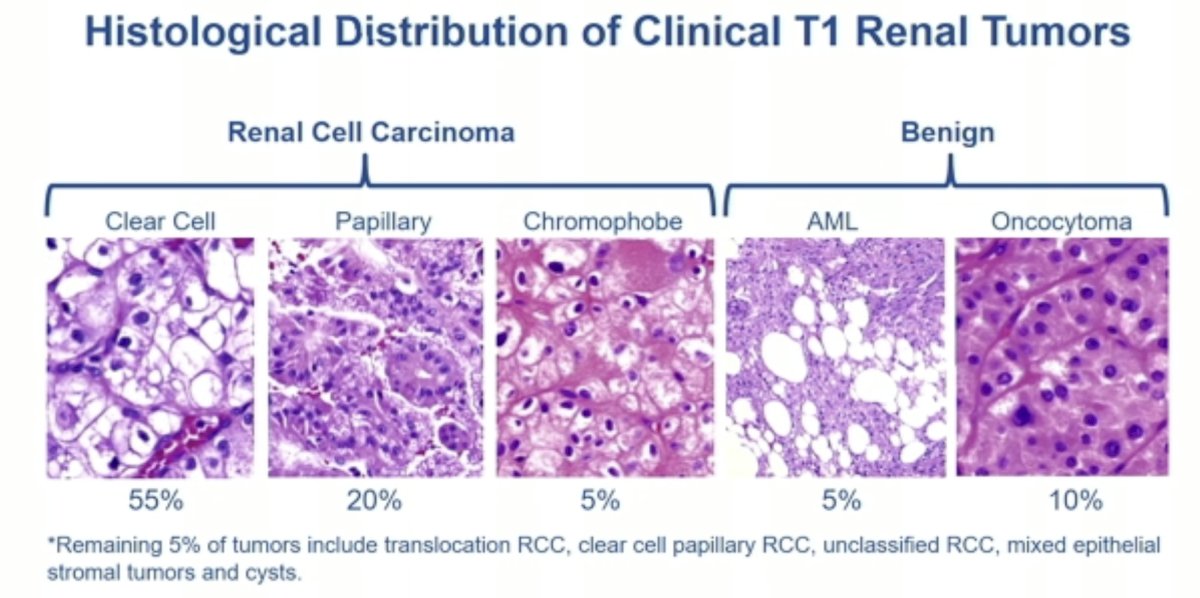
- Differentiating malignant from benign lesions (i.e., renal cell carcinoma from oncocytomas or fat-poor AMLs)
- Differentiating between the RCC subtypes (e.g., chromophobes have more indolent natural histories compared to clear cell lesions)
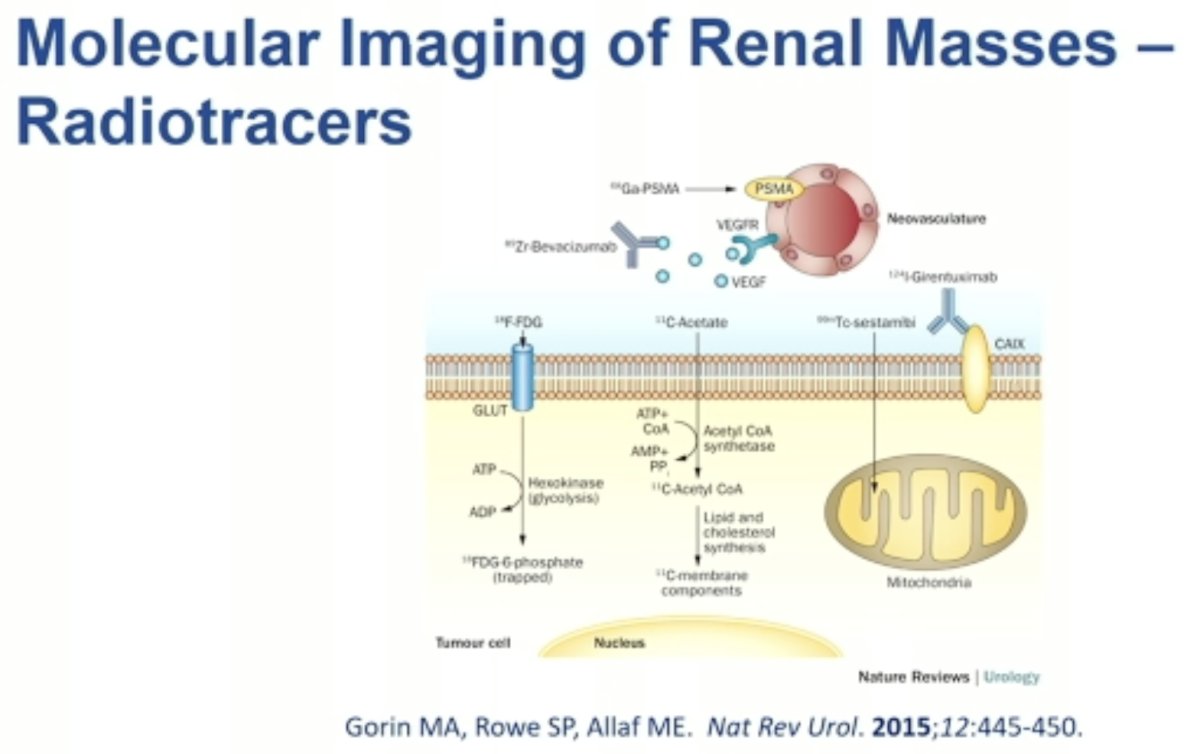
What are some potential targets of molecular imaging radiotracers for renal masses? As illustrated in the schematic below, there are numerous existing/emerging targets that have been evaluated over the past few decades.1
The first molecular imaging modality discussed was 18F-FDG PET/CT of renal masses. Both benign and malignant primary renal masses demonstrate variable uptake of 18F-FDG. This imaging tool performs poorly for the primary lesion characterization; however, it performs more reliably in the metastatic setting whereby there is ~80% uptake in metastatic sites.2 Nevertheless, this tool has a very limited role in the routine evaluation or staging of RCC, mainly owing to its poor specificity (i.e., uptake by normal tissue and other malignancies).

11C-Acetate has been evaluated as a radiopharmaceutical target for the identification of fat-poor angiomyolipomas.
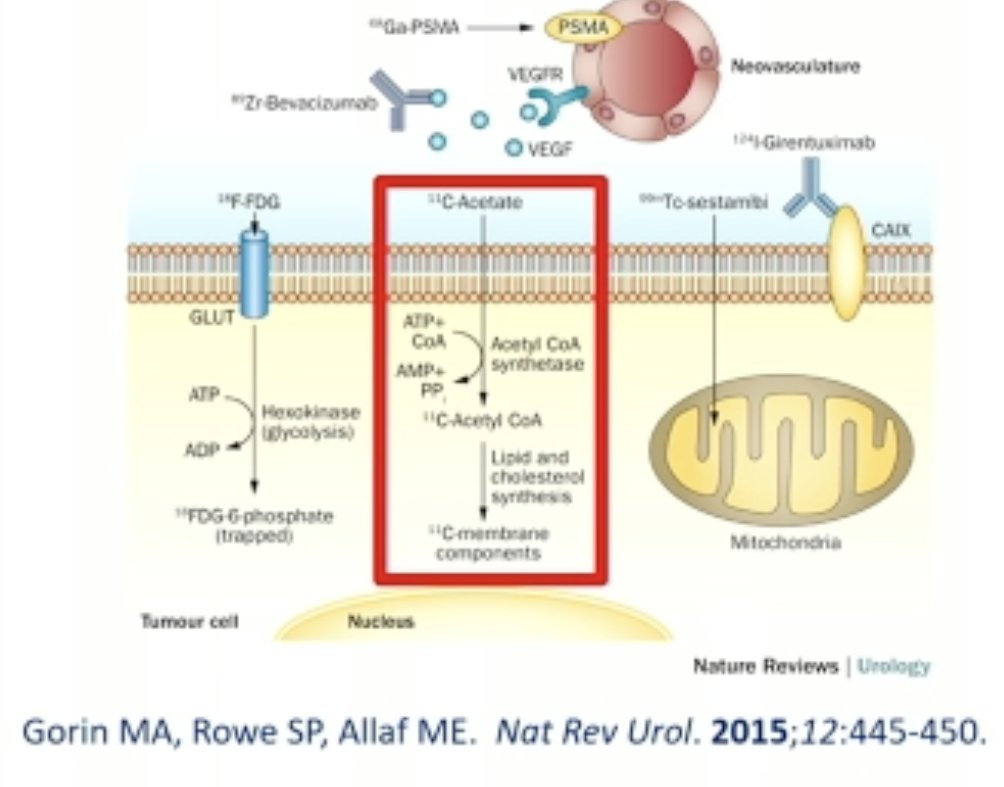
Using the combination of 18F-FDG and 11C-acetate PET/CT, Ho and colleagues evaluated their combined diagnostic performance for differentiating fat-poor angiomyolipomas from RCC. All angiomyolipomas showed negative 18F-FDG but markedly increased 11C-acetate metabolism, significantly higher than RCC (11C-acetate SUVmax ratio = 4.11 ± 0.53 vs 2.00 ± 0.71; p<0.05). 11C-acetate SUVmax ratio = 3.71 could differentiate angiomyolipoma including "fat-poor angiomyolipoma" (n = 10) from RCC with a sensitivity of 94% and specificity of 98%.3

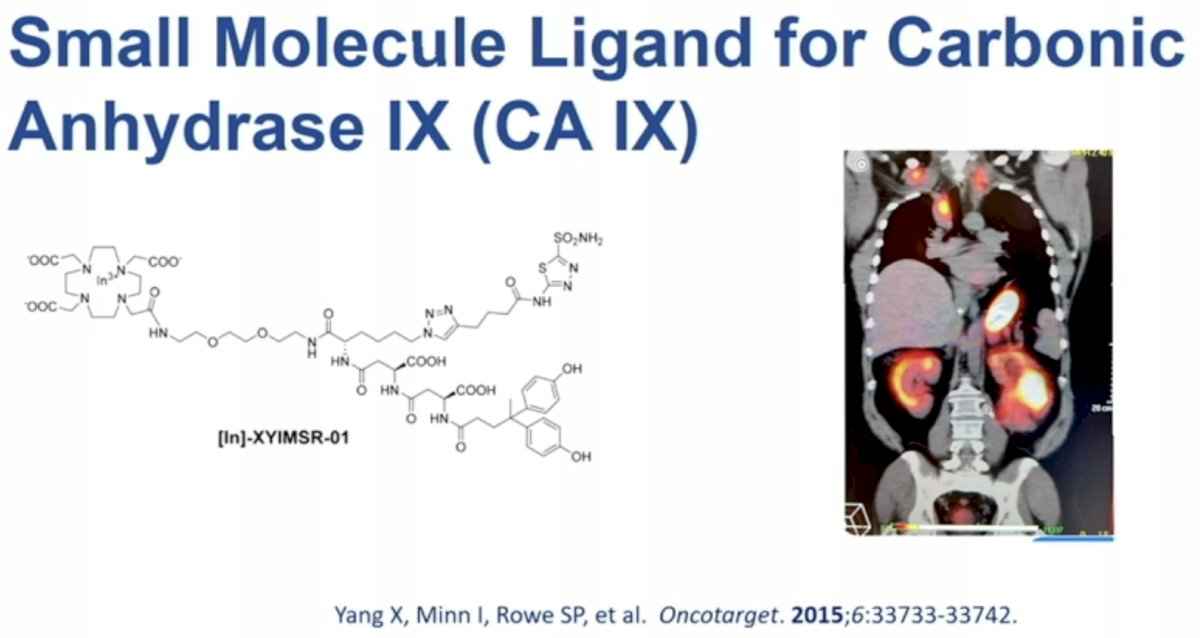
More recently, carbonic anhydrase IX (CAIX) has been ‘re-evaluated’ as a target for renal mass imaging.
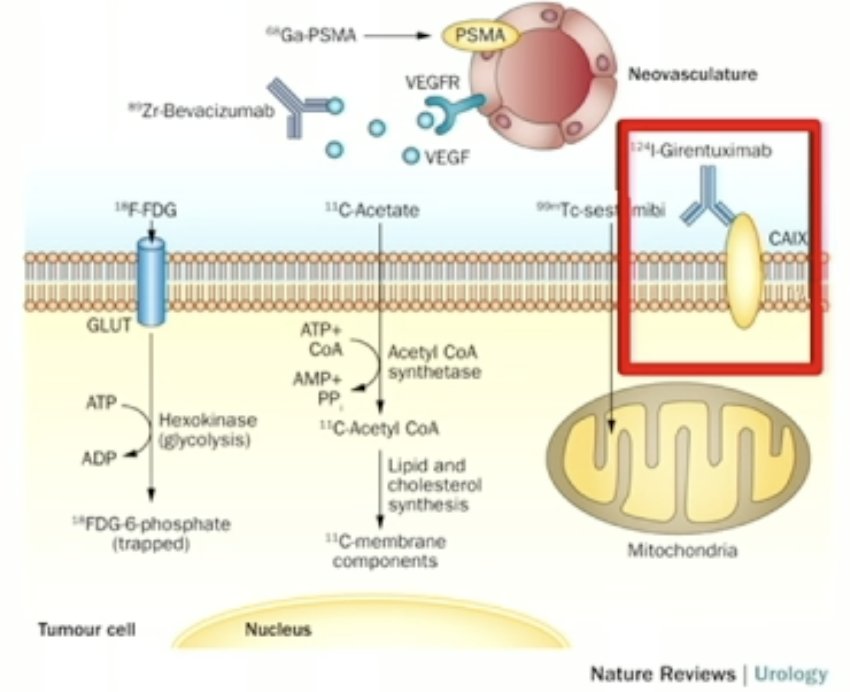
In 2013, Divgi et al. published the results of an open-label multicenter study of iodine-124 ((124)I) -girentuximab PET/CT in patients with renal masses who were scheduled for resection. PET/CT and contrast-enhanced CT (CECT) of the abdomen was performed 2 to 6 days after intravenous (124)I-girentuximab administration and before resection of the renal mass(es). The average sensitivity of PET/CT was 86% (versus 76% for CECT) and the specificity was 86% (versus 47% for CECT). This study represented the first clinical validation of a CAIX-targeting radiotracer for PET/CT imaging for the accurate and non-invasive identification of clear cell RCC.4
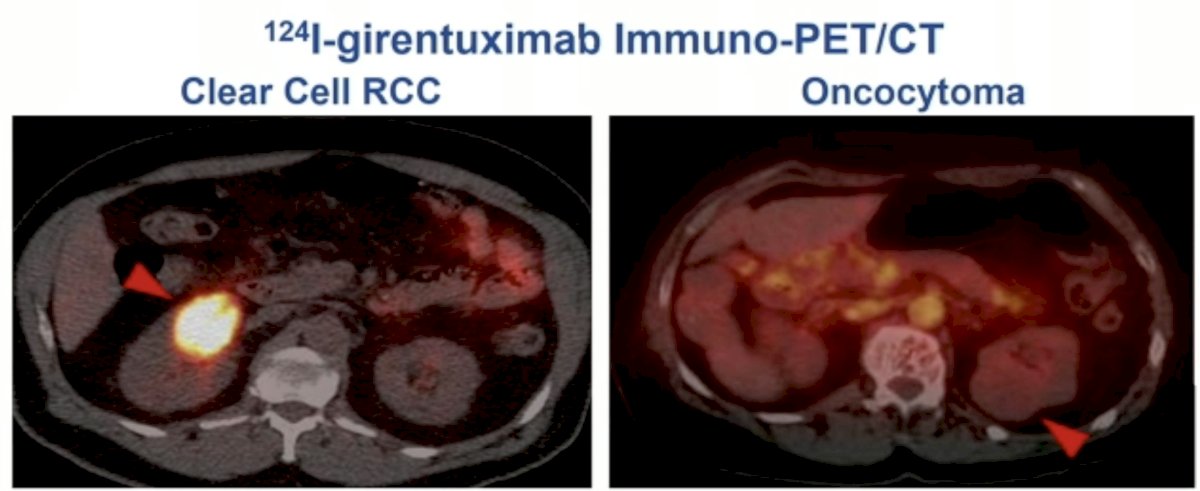
More recently, at GU ASCO 2023, Schuch et al. presented the results of the ZIRCON trial, which evaluated the diagnostic performance of 89Zr-DFO-girentuximab (TLX250-CDx) PET/CT. ZIRCON was an open-label, multicenter clinical trial. Patients with indeterminate renal masses (≤ 7 cm; tumor stage cT1) who were scheduled for a partial or radical nephrectomy within 90 days from planned 89Zr-DFO-girentuximab administration were eligible. Enrolled patients received a single dose of 89Zr-DFO-girentuximab (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery:
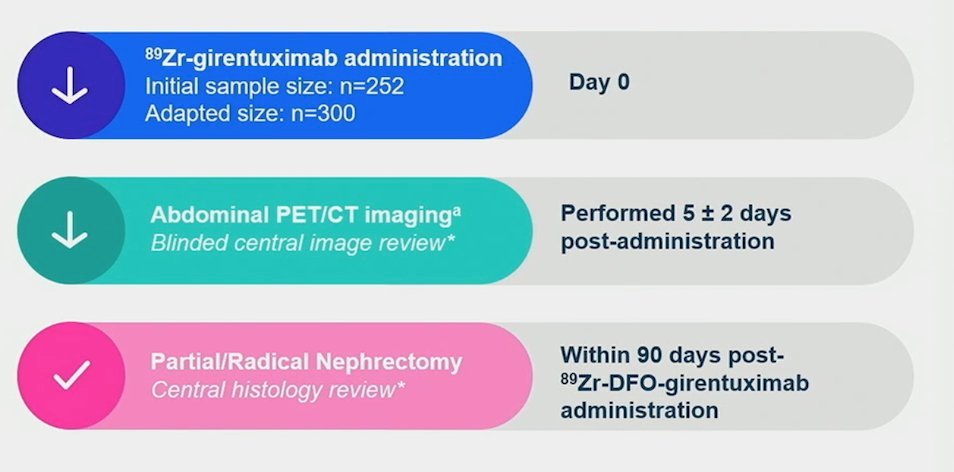
This imaging modality demonstrated:
- Sensitivity: 86%
- Specificity: 87%
- PPV: 91%
- NPV: 74%

Additional CAIX-targeting small molecule ligands are under evaluation, including [In]-XYIMSR-01.
99mTc-Sestamibi scanning for renal mass imaging has long been evaluated for differentiating oncocytomas from RCCs.

99mTc-Sestamibi undergoes intracellular uptake by mitochondria leading to its accumulation in these intracellular structures.
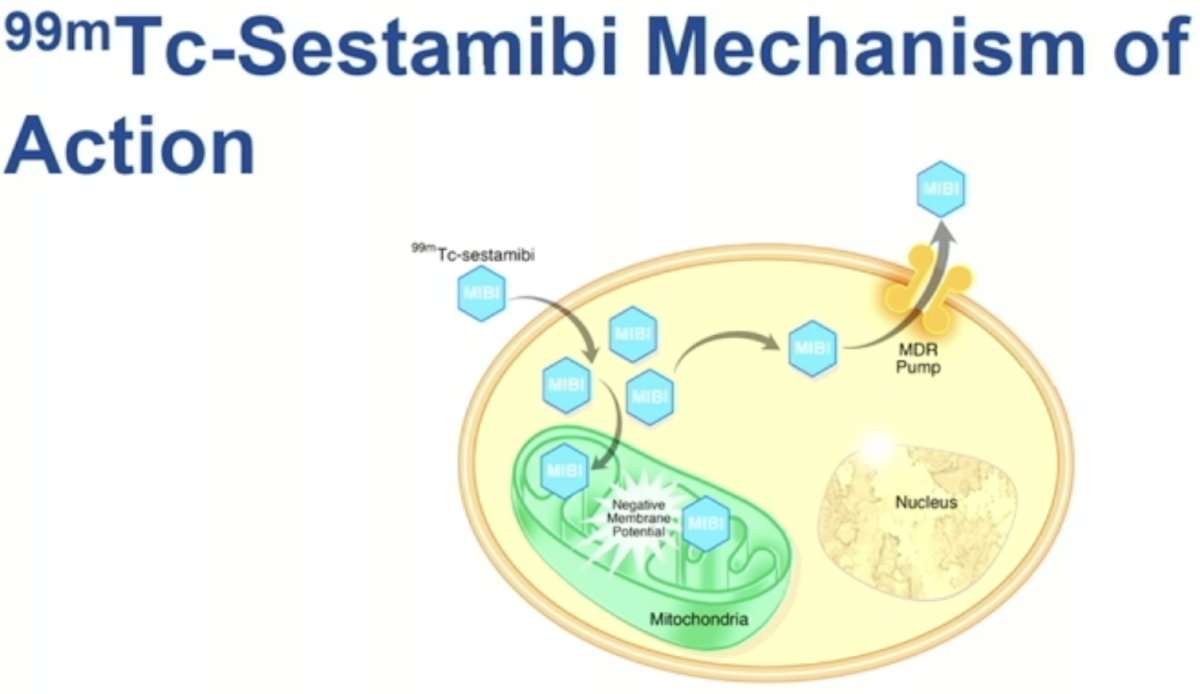
Clear cell RCCs are mitochondria poor. Conversely, oncocytomas demonstrate higher intracellular concentrations of mitochondria. As such, these lesions demonstrate increased uptake of 99mTc-Sestamibi.
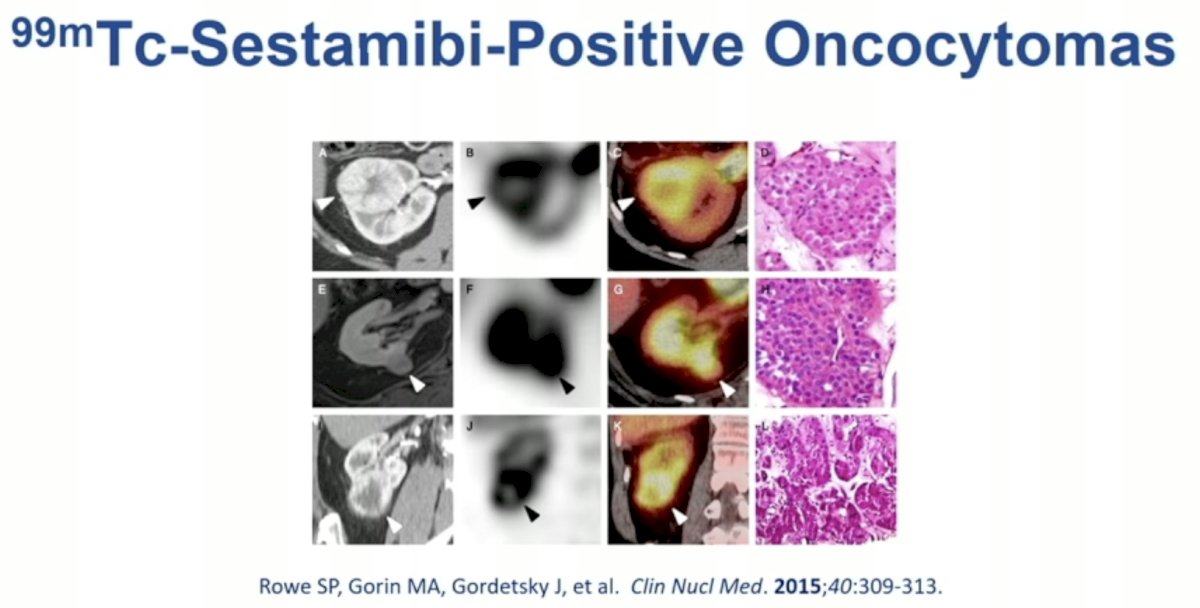
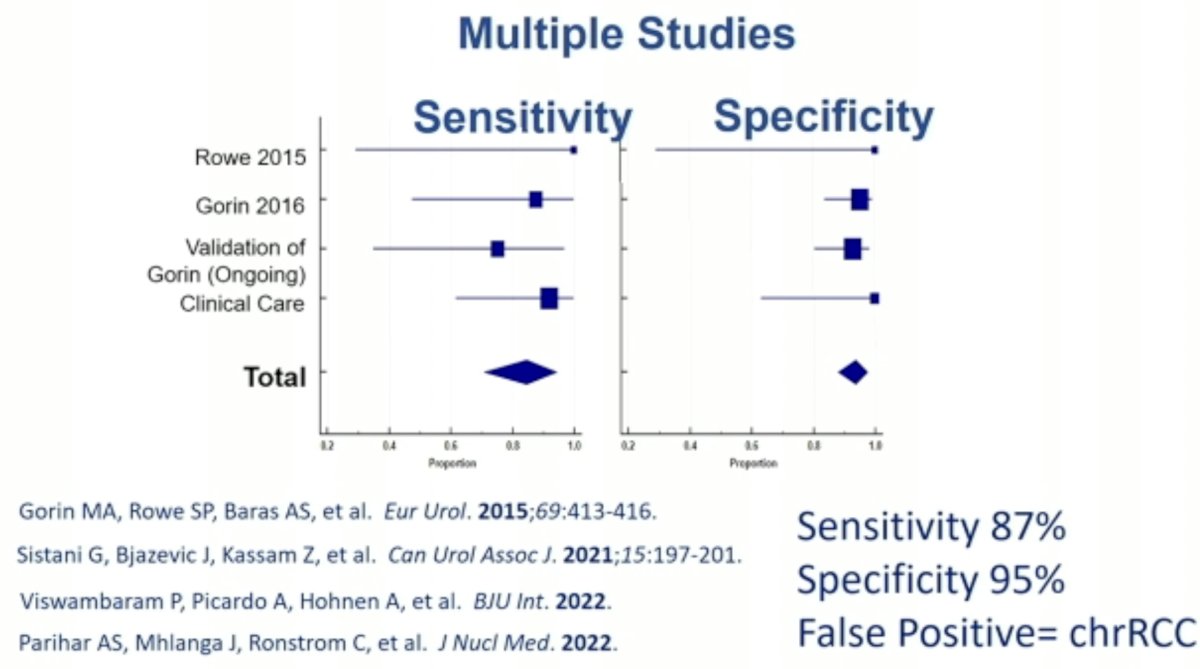
In 2016, Dr. Allaf’s group published the results of a meta-analysis evaluating the diagnostic performance of 99mTc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. This modality demonstrated a sensitivity of 87% and specificity of 95%. The most common false positive lesions in this setting are chromophobe RCC.5
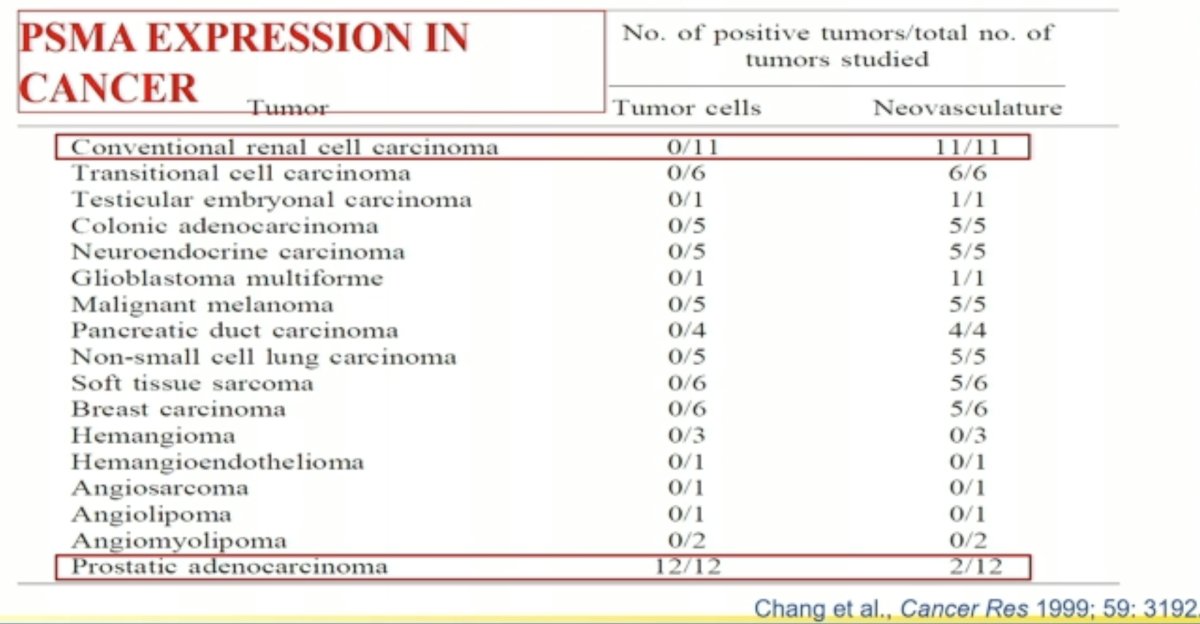
PSMA-targeted imaging has also emerged in the metastatic RCC setting. However, in contrast to prostate cancer, where PSMA is expressed on the cancerous cell surface, PSMA is not expressed by conventional RCC tumor cells. It is instead expressed in the renal neovasculature.
Numerous reports to date have demonstrated the utility of PSMA-PET/CT for the staging of metastatic RCC patients. PSMA-based PET/CT with radiotracers such as ¹⁸F-DCFPyL may allow more accurate staging of patients with RCC and conceivably the ability to predict and follow therapy in patients treated with agents targeting the neovasculature,
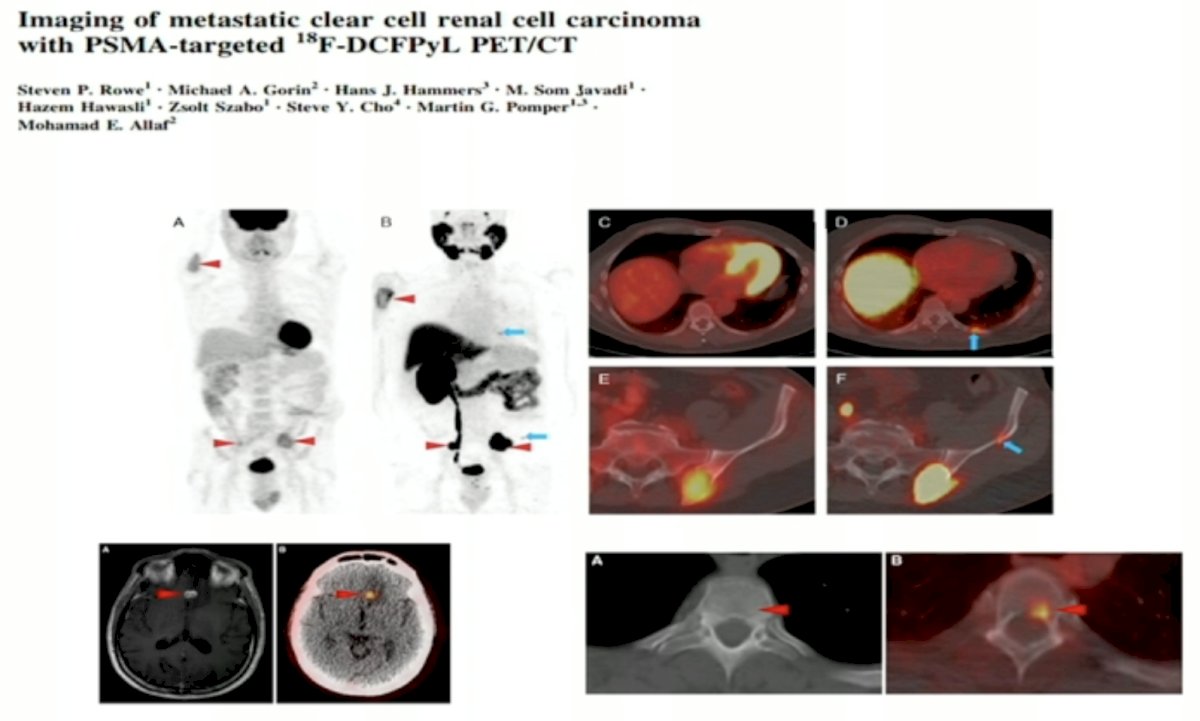
Dr. Allaf noted that PSMA PET/CT may detect sites of unusual metastasis, including triceps metastasis, as illustrated below:

Future directions in this space may include the use of dual tracer imaging, such as combined 99mTc-sestamibi (differentiate oncocytomas from RCCs) and 111In-CAIX SPECT/CT (to differentiate clear cell RCC from other subtypes), which may change the current work-up of small renal masses, including omitting renal mass biopsies.
Dr. Allaf concluded his presentation as follows:
- Many molecular imaging agents exist for renal tumors and many more are in development
- The clinical utility of these agents is being defined
- Many variables are at play: SPECT vs. PET; antibody vs. peptide vs. small molecule; diagnostic vs. staging vs. response to therapy
- The role of radiomics and artificial intelligence will likely enhance utility
- Imaging agents are usually precursors for theranostic agents
Presented by: Mohamad Allaf, MD, Professor of Urology, Urologist-in-Chief, The Johns Hopkins Hospital, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Gorin MA, Rowe SP, Allaf ME. Nuclear imaging of renal tumours: a step towards improved risk stratification. Nat Rev Urol. 2015;12(8): 445-50.
- Wang HY, Ding HJ, Chen JH, et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging. 2012;12(3): 464-74.
- Ho CL, Chen S, Ho KMT, et al. Dual-tracer PET/CT in renal angiomyolipoma and subtypes of renal cell carcinoma. Clin Nucl Med. 2012;37(11): 1075-82.
- Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol. 2013;31(2): 187-94.
- Gorin MA, Rowe SP, Baras AS, et al. Prospective Evaluation of (99m)Tc-sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur Urol. 2016;69(3): 413-6.


