(UroToday.com) Dr. Abhinav Khanna and his Mayo Clinic team presented a captivating study on postoperative renal function assessment following extirpative renal surgeries. Long-term kidney function post radical nephrectomy (RN) or partial nephrectomy (PN) heavily relies on baseline pre-operative kidney function, typically measured by estimated glomerular filtration rate (eGFR), and the preserved parenchyma during surgery. However, saving renal parenchyma poses a risk factor for chronic kidney disease, making its quantification challenging post-surgery. The surgeon's objective is to conserve as much non-neoplastic tissue as feasible to retain the patient's post-operative renal function as close as possible to its pre-interventional state. Therefore, it underscores the significance of a method to quantify kidney function post-operatively. Despite suggestions for renal volume comparisons via cross-sectional imaging, the need for a more efficient system persists. To address this, Dr. Khanna and his team pursued two primary objectives: first, to develop a deep learning algorithm capable of detecting and quantifying renal volume, specifically non-neoplastic volume, utilizing contrast-enhanced CT scans. Second, they aimed to correlate the automated renal volume estimates with post-operative renal function (PORF) following extirpative renal surgeries and to automate the calculation of non-neoplastic renal volumes using only patient pre-operative CT scans.
In this retrospective study, patients who underwent either RN or PN at a Mayo Clinic tertiary referral center within the last decade were identified. All patients needed to have accessible pre-operative CT images. Leveraging nnU-Net architecture, Dr. Khanna and his team developed a novel deep learning algorithm designed to automatically measure the ipsilateral renal volume (RV), contralateral RV, and kidney tumor volume on contrast-enhanced CT scans (see figure below)
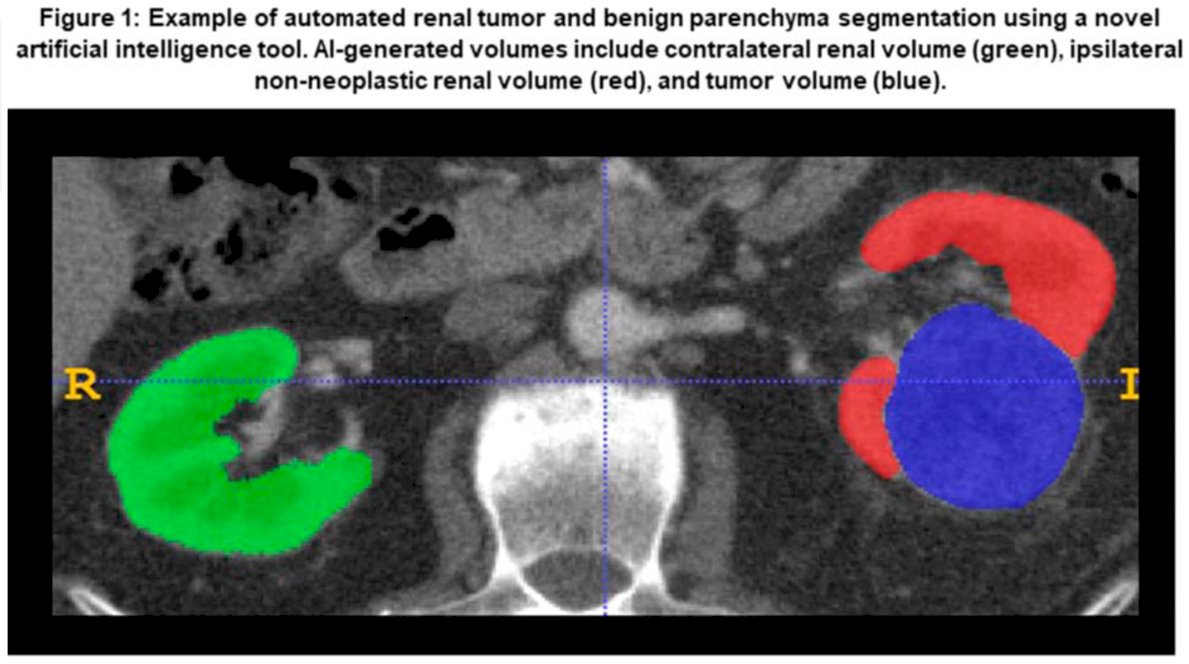
The relationships between algorithm-generated pre-operative non-neoplastic RV and observed PORF were evaluated using generalized linear mixed-effect models, controlling for established clinical factors linked to PORF, such as age, diabetes, pre-operative eGFR, tumor size, and time from surgery.
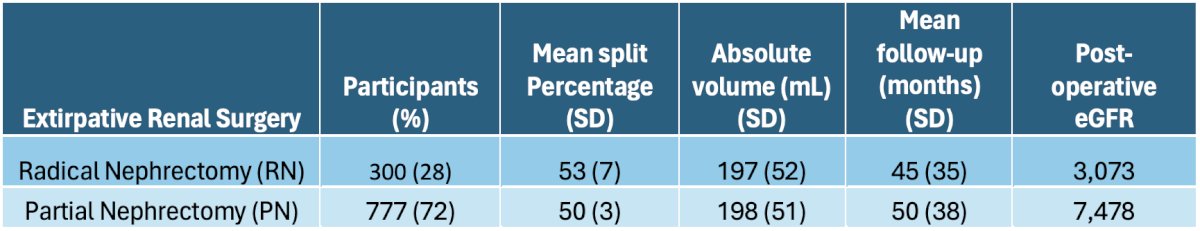
The study cohort comprised 1,077 patients, including 300 (28%) RN and 777 (72%) PN cases. Among RN patients, the contralateral RV exhibited a mean split percentage of 53% (SD=7), with an absolute volume of 197 mL (SD=52). These patients had a mean follow-up duration of 45 months (SD=35), during which 3,073 post-operative eGFR assessments were conducted. For PN patients, the mean split percentage was 50% (SD=3), with an absolute volume of 198 mL (SD=51). PN patients were followed up for a mean duration of 50 months (SD=38), with 7,478 post-operative eGFR assessments. On average, each patient underwent 10 (SD=9) post-operative eGFR measurements (refer to the table below).
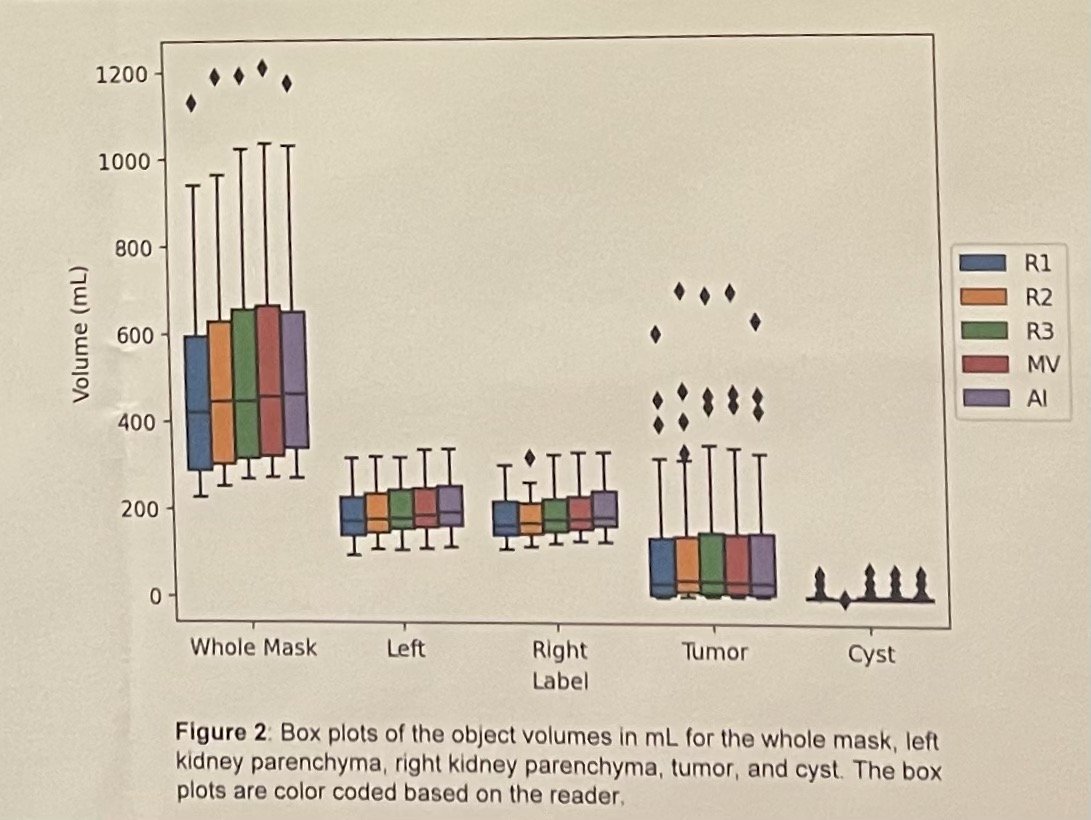
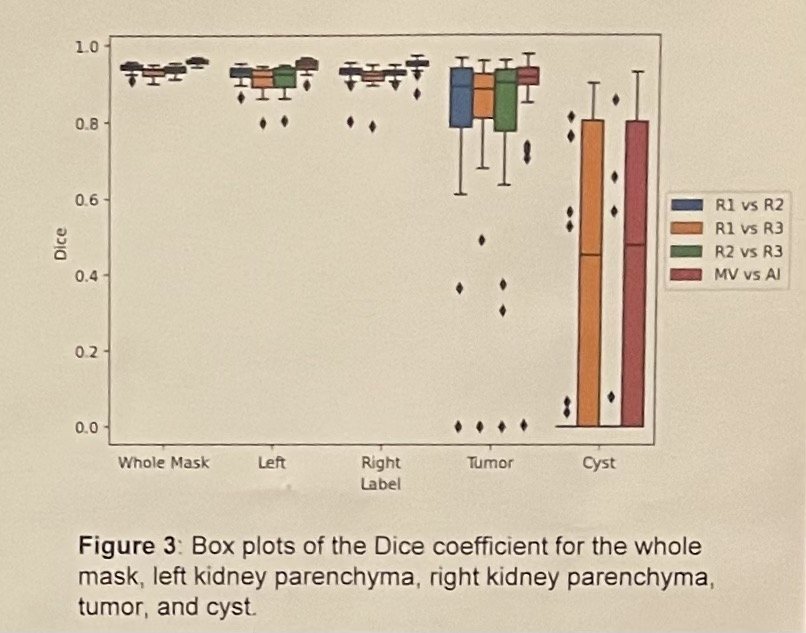
Dr. Khanna's innovative deep learning algorithm demonstrated remarkable accuracy in identifying renal volume (RV) compared to human assessments (DICE=0.94) (see figures below). Additionally, it effectively distinguished ipsilateral RV from the tumor. Statistical analysis, employing a validated multivariable clinical model to predict PORF while adjusting for known variables associated with long-term kidney function, revealed a significant correlation between elevated AI-derived contralateral RV and improved PORF outcomes in both RN (p<0.001) and PN (p<0.001) cases. Furthermore, each 10% increase in split contralateral RV corresponded to a PORF increase of 6.5 mL/min/1.73 m2 in RN patients and 2.6 mL/min/1.73 m2 in PN patients.
In conclusion, Dr. Khanna underscored the association between pre-operative non-neoplastic RV and long-term renal function, evident in both RN and PN cases even after adjusting for a previously validated clinical prediction model. Significantly, his team developed a learning algorithm capable of computing automated RV assessments using artificial intelligence solely from CT images. Dr. Khanna suggested that this novel instrument could facilitate the integration of RV measurements into clinical practice, overcoming current challenges.
During the question-and-answer session, Dr. Francesco Porpiglia raised concerns about how this novel artificial intelligence algorithm compares to other renal function assessment tools, given that volume and function may not always align. Specifically, Dr. Porpiglia brought up renal scans, the gold standard for assessing kidney function. He emphasized that to establish the renal function of the operated kidney, a renal scan is indispensable, as it offers a wide variety of topologies that assist the surgeon in assessing kidney functionality after intervention. Dr. Khanna acknowledged the significance of renal scans in assessing kidney function but noted the limited availability of such data in their study. He emphasized ongoing efforts to train the model to predict function without renal scans and to correlate CT-based renal features with renal scan results for improved function assessment.
Presented by: Abhinav Khanna, MD, Mayo Clinic, Rochester, Minnesota
Written by: Seyed Amiryaghoub M. Lavasani, B.A., University of California, Irvine, @amirlavasani_ on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


