(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX was host to the Society of Urologic Oncology (SUO) session Dr. Leslie Ballas in her presentation discussed the role of radiation therapy for localized and oligometastatic renal cell carcinoma (RCC) and delve into future indications for radiation therapy in different spaces of the disease.
Dr. Ballas began her presentation with an overview of the role of radiation therapy (RT) in renal cell carcinoma (RCC). She discussed various indications for RT, encompassing intracranial disease (postoperative, definitive for 1-5 lesions, and whole brain RT after progression), spinal disease (postoperative and palliative), primary RCC (for medically inoperable cases, technically challenging scenarios unsuitable for other nephron-sparing procedures, and larger tumors (>3cm) inappropriate for thermal ablation), as well as salvage post partial nephrectomy or thermal ablation (RFA or cryoablation), and extracranial disease in select patients with oligometastatic disease or oligoprogression to delay systemic treatment switch. She presented 1-year local control rates and Grade 3/4 toxicity data for each indication of RT, as summarized in the figure below.

Dr. Ballas discussed the evolving landscape of stereotactic ablative radiotherapy (SABR) in the management of primary renal cell carcinoma (RCC), noting its recent inclusion in the NCCN 2024 guidelines. According to these guidelines, SABR may be considered for medically inoperable patients with stage I kidney cancer or with stage II/III kidney cancer. She delved into the evidence base supporting SABR for primary RCC, citing a recent review that analyzed SABR outcomes. This review encompassed two meta-analyses comprising 54 studies, along with 13 prospective and 20 retrospective studies. The median tumor size ranged from 3.2 to 5.6 cm, and the delivered dose (Gy) and fractions varied from 30-40 Gy divided over 3-7 fractions. Local control rates were consistently high, ranging from 92% to 97%. Further details are provided in the table below.

Dr. Ballas proceeded to discuss the IROCK consortium meta-analysis. This study entailed an individual patient data meta-analysis involving patients undergoing SABR for primary RCC across five countries (Australia, Canada, Germany, Japan, and the USA). SABR was administered either as a single fraction or multiple fractions of greater than 5 Gy. The primary endpoint was investigator-assessed local failure per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Data from 190 patients who received SABR were presented, with almost half of them receiving single-fraction SABR (43%) and the remaining undergoing multifraction SABR. The median tumor diameter was 4.0 cm (IQR 2.8–4.9), and 75% of patients with available operability details were deemed inoperable by the referring urologist. Approximately a third of patients had a solitary kidney. She then proceeded to illustrate that at 5 years post-SABR, the cumulative incidence of local failure was 5.5% (Figure below) with single-fraction SABR resulting in fewer local failures than multifraction. The toxicity observed in the patients included in this meta-analysis was low, and SABR was well tolerated, with no grade 3 toxic effects or treatment-related deaths reported.
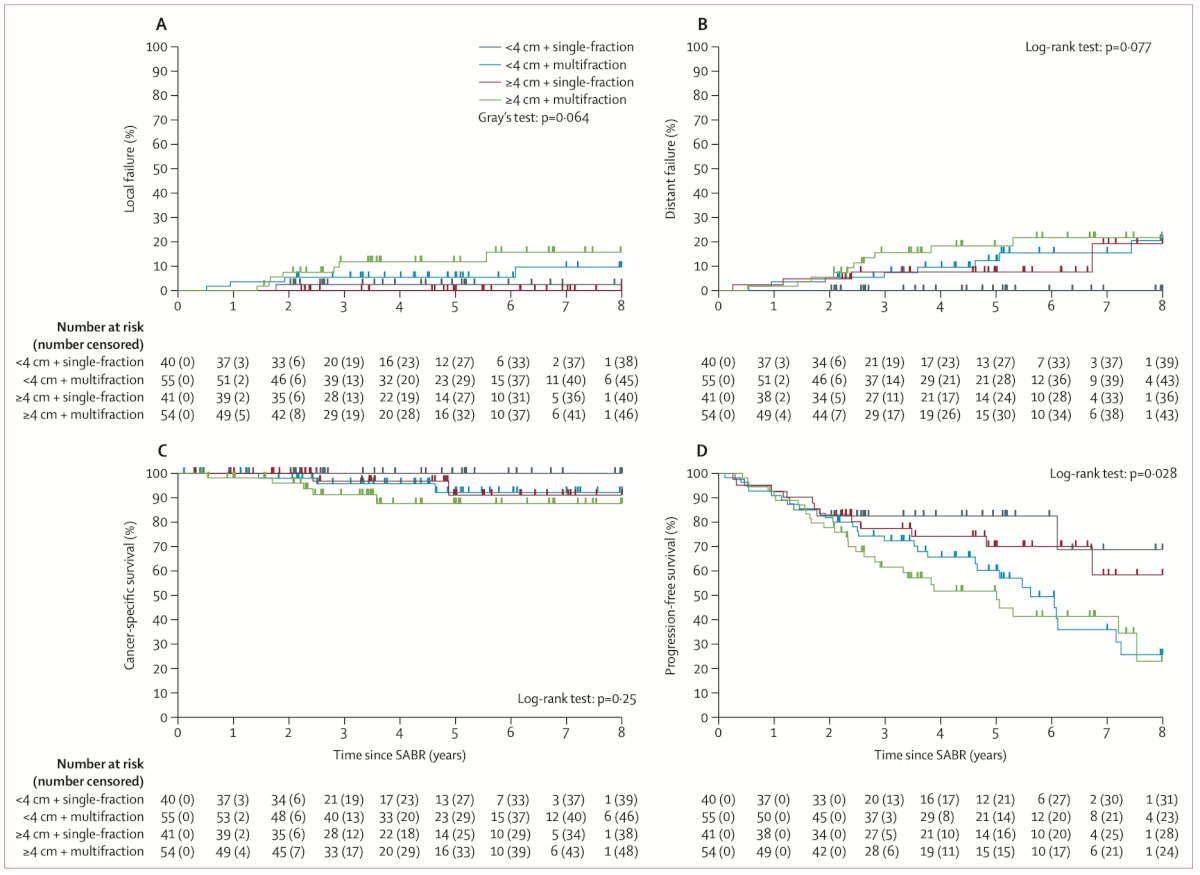
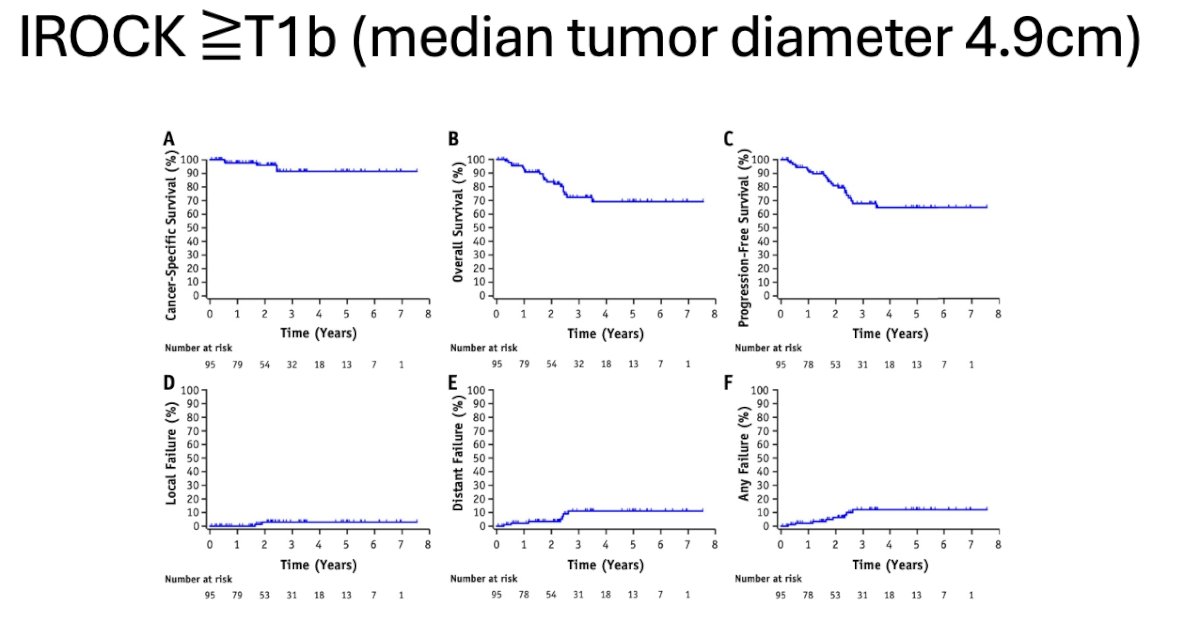
She elaborated that in patients with stage T1b or greater (median diameter 4.9 cm) included in the IROCK meta-analysis, cancer-specific survival, overall survival, progression-free survival, and local failure rate did not differ from those of other patients. This subset of patients could also be considered amenable to receive treatment with SABR. (Figure below)
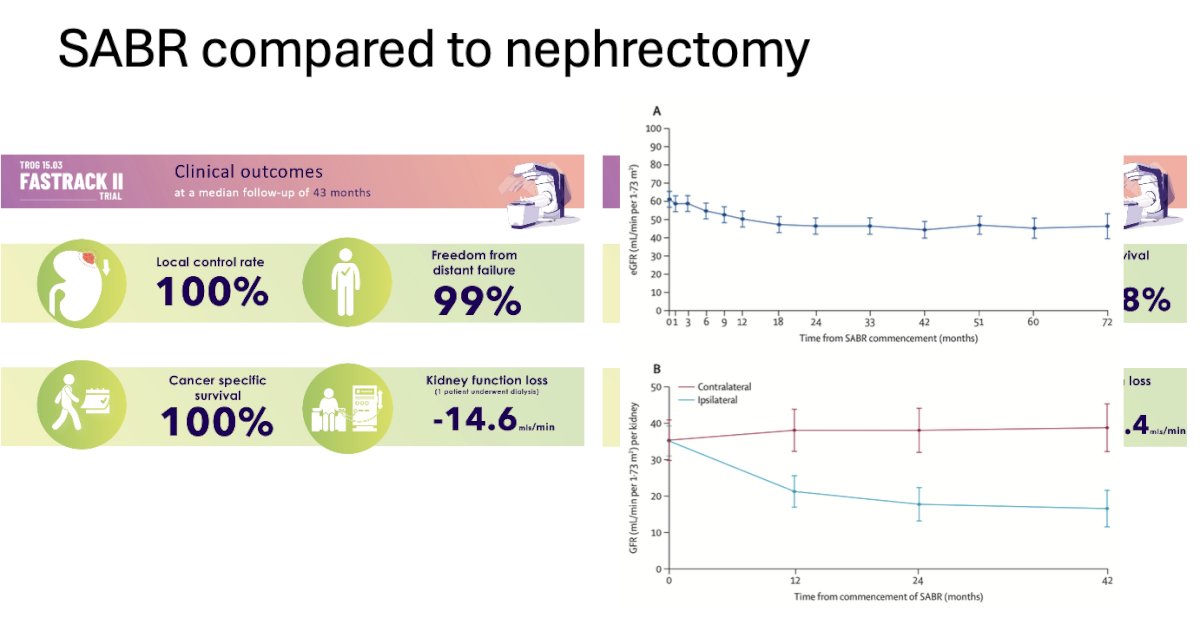
Dr. Ballas discussed the TROG 15.03 FASTRACK II trial, the first multicenter phase II trial of non-surgical therapy for primary RCC. It included patients with biopsy-confirmed primary RCC having a single lesion within the kidney, deemed inoperable or high-risk for surgery, with a multidisciplinary decision that active treatment is warranted, and the tumor being <10 cm and not abutting the bowel. The aim was to evaluate local control after SABR, with local control ≤80% considered not worthy of proceeding to a future randomized controlled trial. At a median follow-up of 43 months, the trial demonstrated a local control rate of 100%, freedom from distant failure of 99%, and 100% cancer-specific survival. Interestingly, SABR was associated with a median loss in kidney function (eGFR) of -14.6 ml/min
Dr. Ballas then transitioned to the metastatic RCC space, presenting the ASCO guideline. According to this guideline, RT is recommended for patients with low-volume metastatic disease, bone metastasis (if symptomatic), and brain metastasis. She proceeded to discuss SABR for oligometastatic RCC, noting that three prospective and two retrospective trials exploring SABR in oligometastatic disease have shown that SABR may delay the need for systemic treatment without compromising survival. The percentage of patients free from systemic treatment at 1 year in these studies ranged from 70% to 91%. She presented data from a phase 2 trial exploring SABR in systemic therapy-naïve oligometastatic RCC. In this trial, the freedom from systemic therapy at one year was 91.3%, and toxicity was low. However, she also mentioned that there could be a role for SABR in the oligoprogressive space, but further research is needed in this field.

She concluded by emphasizing that SABR for primary RCC has demonstrated outstanding efficacy and safety, emerging as a new option for inoperable RCC and oligometastatic RCC. Moreover, she highlighted ongoing trials, such as the NRG-GU 012: SAMURAI trial, which explores cytoreduction in metastatic RCC by comparing standard immunotherapy (IO) versus IO + SABR (42 Gy).
Presented by: Leslie Ballas MD, Radiation Oncologist at Cedars Sinai Medical Center, Los Angeles, California
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Correa RJM, Louie AV, Zaorsky NG, Lehrer EJ, Ellis R, Ponsky L, Kaplan I, Mahadevan A, Chu W, Swaminath A, Hannan R, Onishi H, Teh BS, Muacevic A, Lo SS, Staehler M, Siva S. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur Urol Focus. 2019 Nov;5(6):958-969. doi: 10.1016/j.euf.2019.06.002. Epub 2019 Jun 24. PMID: 31248849.
- Ali M, Mooi J, Lawrentschuk N, McKay RR, Hannan R, Lo SS, Hall WA, Siva S. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur Urol. 2022 Dec;82(6):613-622. doi: 10.1016/j.eururo.2022.06.017. Epub 2022 Jul 14. Erratum in: Eur Urol. 2022 Nov;82(5):e152. PMID: 35843777.
- Siva S, Ali M, Correa RJM, Muacevic A, Ponsky L, Ellis RJ, Lo SS, Onishi H, Swaminath A, McLaughlin M, Morgan SC, Cury FL, Teh BS, Mahadevan A, Kaplan ID, Chu W, Grubb W, Hannan R, Staehler M, Warner A, Louie AV. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022 Dec;23(12):1508-1516. doi: 10.1016/S1470-2045(22)00656-8. Epub 2022 Nov 16. PMID: 36400098.
- Hannan R, McLaughlin MF, Pop LM, Pedrosa I, Kapur P, Garant A, Ahn C, Christie A, Zhu J, Wang T, Robles L, Durakoglugil D, Woldu S, Margulis V, Gahan J, Brugarolas J, Timmerman R, Cadeddu J. Phase 2 Trial of Stereotactic Ablative Radiotherapy for Patients with Primary Renal Cancer. Eur Urol. 2023 Sep;84(3):275-286. doi: 10.1016/j.eururo.2023.02.016. Epub 2023 Mar 8. PMID: 36898872; PMCID: PMC10440291.


