(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a male incontinence session, and a presentation by Dr. Emmanuel Chartier-Kastler discussing the first results of the SOPHIA study using the new artificial urinary sphincter UroActive™. UroActiveTM is an implantable artificial urinary sphincter made of an occlusive cuff connected to a control unit which includes a reservoir, a pump, a battery, and electronic components able to communicate wirelessly. The occlusive cuff is placed around the bulbar urethra and the control unit is placed in the right part of the abdomen above the aponeurosis. During follow-up visits, the clinician can download data recorded by the control unit and set device parameters. The patient can open the cuff to void and decrease device pressure when lying down using a dedicated remote control. A safety function named UroTimerTM automatically deactivates the device if the patient does not void during a certain period. The clinician is able to program the settings of the implant, for example: baseline pressure (ie. 60 cmH20), lying down pressure (ie. 10 cm H20), UroTimerTM period (ie. 12 hours). Dr. Chartier-Kastler notes that UroactiveTM is neither CE Marked nor FDA approved yet:
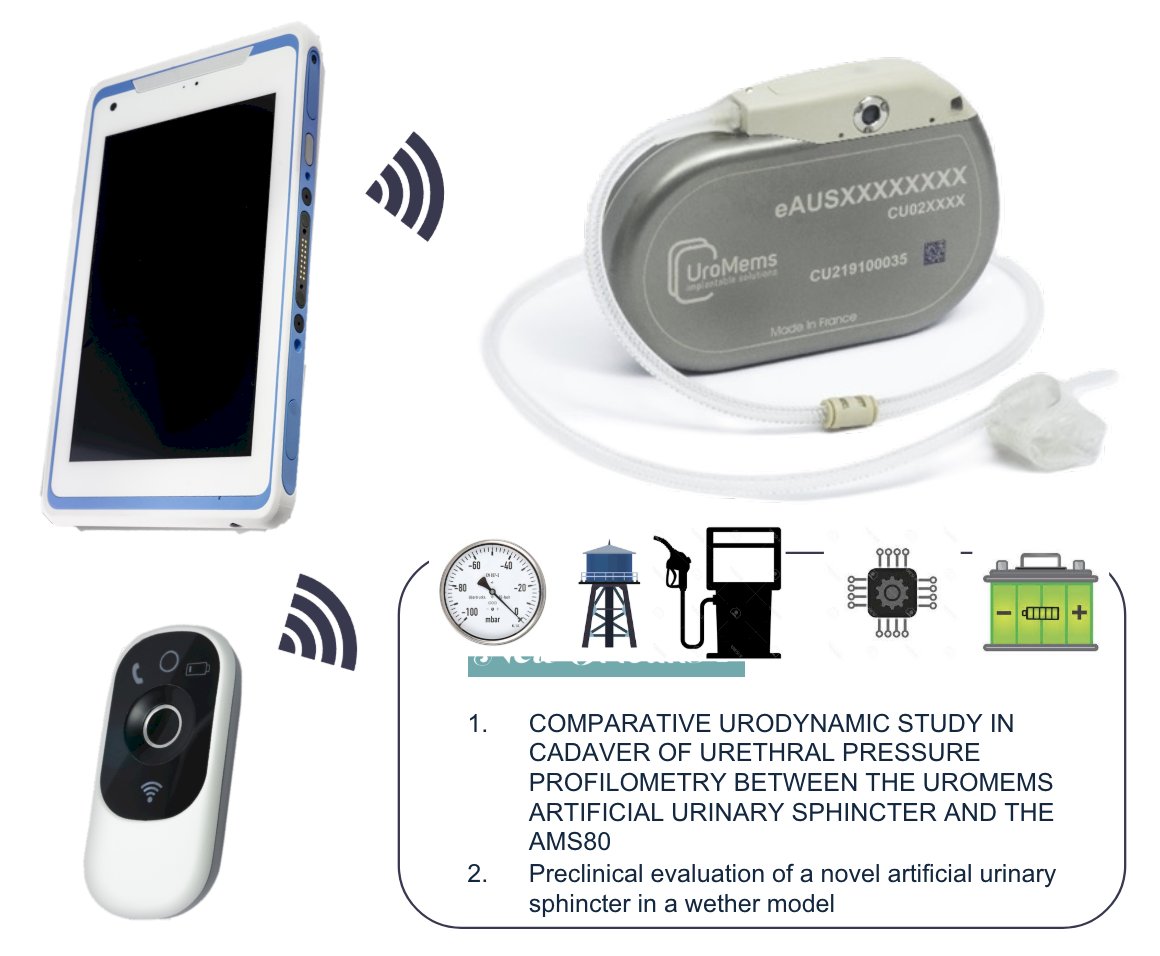
The SOPHIA study was a prospective, open-label, multi-center, single-arm study designed to assess the safety and the effectiveness of UroActive™ in 6 patients (NCT05547672). After the implantation and a healing period of 5 weeks, the device is activated. Follow-up visits were planned at day 14, as well as 1, 3, and 6 months post-activation to adjust device parameters according to patient feedback on continence:

The primary clinical outcomes were the rate of explants and revisions at 6 months after device activation, and the rate of device activation successes. The main secondary clinical outcomes were:
- Number of subjects with 50% reduction or greater in 24-hour pad weight test at 3 and 6 months after device activation
- Number of subjects with 75% reduction or greater in 24-hour pad weight test at 3 and 6 months after device activation
- Quality of life questionnaires (ICIQ-UI SF, ICIQ-MLUTS, EQ-5D3L, I-QoL, and USP) at baseline, 3 months, and 6 months post-activation
Among the 6 patients enrolled in SOPHIA, the median age was 71 years (IQR 66-73), median BMI was 25.3 kg/m2 (IQR 24.5-26.4), all had a sphincter deficiency after radical prostatectomy, no patient had prior radiotherapy, and one patient had a history of previous stress urinary incontinence surgery. All devices were successfully implanted and activated, and no explanations or revisions were required. The median implantation time was 64 minutes (IQR 61-69), with one severe adverse event including a post-operative hematoma with dysuria (self-resolved). At 3 months after device activation, all patients had a 24-hour pad weight test reduction of more than 50% (median 86%, IQR 78-91), with 5 patients having a 24-hour pad weight test reduction of more than 75%. Additionally, at 6 months after device activation, all patients had a 24-hour pad weight test reduction of more than 50% (median 82%, IQR 76-92), with 5 patients having a 24-hour pad weight test reduction of more than 75%. At 6 months, the median Qmax was 36 mL/s (IQR 25-41) and median post-void residual was 0 mL (IQR 0-5). UroTimerTM safety function triggered three times because of function setting (time too short) and once following an adverse event unrelated to the device. All devices were reactivated without difficulties.
Dr. Chartier-Kastler concluded his presentation by discussing the first results of the SOPHIA study using the new artificial urinary sphincter UroActive™ with the following take-home messages:
- The primary and secondary outcomes were reached in this study with more than one year of follow-up for four patients
- A multicenter and international pivotal clinical trial is planned to start
- The first in women clinical trial is on the way (NCT05828979)
Presented by: Emmanuel Chartier-Kastler, MD, PhD, Sorbonne Université, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


