(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to an advanced prostate cancer podium session. Dr. Stephen Freedland presented the results of a post hoc analysis of EMBARK evaluating PSA dynamics.
On November 16, 2023, the United States Food and Drug Administration (FDA) approved enzalutamide for the treatment of non-metastatic castration-sensitive prostate cancer patients with biochemical recurrence at high risk for metastasis, based on results of the EMBARK trial (NCT02319837).1,2 EMBARK showed significant and clinically meaningful improvements in metastasis-free survival with enzalutamide + leuprolide combination therapy (p<0.001) and enzalutamide monotherapy (p<0.005), compared to placebo + leuprolide, while maintaining high health-related QoL in patients with high-risk biochemical recurrence.3 A key feature of EMBARK was that patients were treated for 36 weeks, followed by treatment suspension at week 37 if PSA levels at week 36 were undetectable (<0.2 ng/ml) and reinitiated if PSA levels rose to protocol-defined thresholds. The objective of this analysis was to present a post hoc analysis of PSA dynamics in the EMBARK trial to understand the time course to undetectable PSA and the likelihood of undetectable PSA levels after treatment re-initiation.
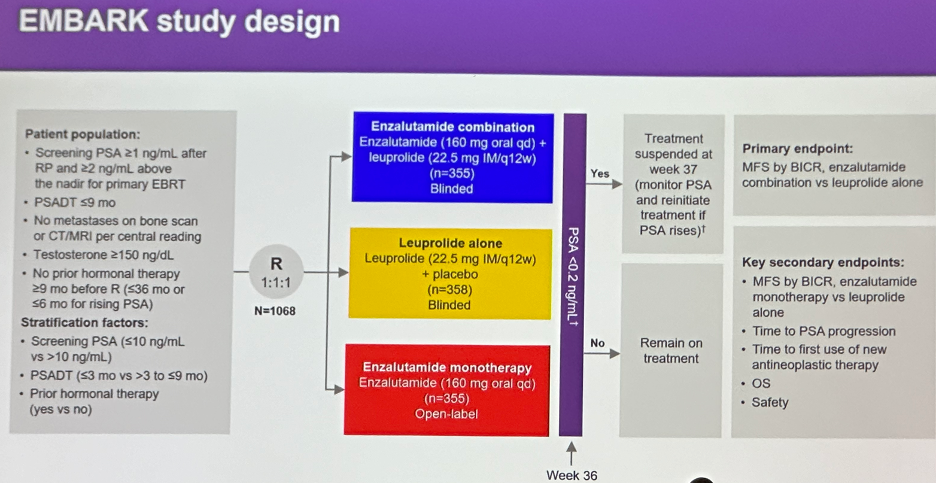
The EMBARK study design is illustrated below. In brief, all patients had a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir after primary external beam radiotherapy, with a PSA doubling time (PSADT) of ≤9 months. Patients had no evidence of metastasis on conventional imaging and the baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSADT, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide acetate (blinded arm)
- Placebo + leuprolide acetate (blinded)
- Enzalutamide monotherapy (unblinded)
PSA was assessed at 36 weeks, and if patients had:
- PSA<0.2 Treatment was suspended at week 37 and PSA was monitored with treatment reinitiated if PSA rose again
- PSA>0.2 Treatment was continued
Baseline characteristics are summarized below. Median age was about 69 years, with the majority of patients Caucasian (83%). Consistent with the stratified randomization technique, about 20% of patients in each arm had a PSADT ≤3 months, with the remaining 80% having a PSADT of 3 to 9 months. The median PSADT was approximately 5 months. 30% of patients had received prior hormonal therapy. Of note about half of the cohort had received both prior radical prostatectomy and radiotherapy.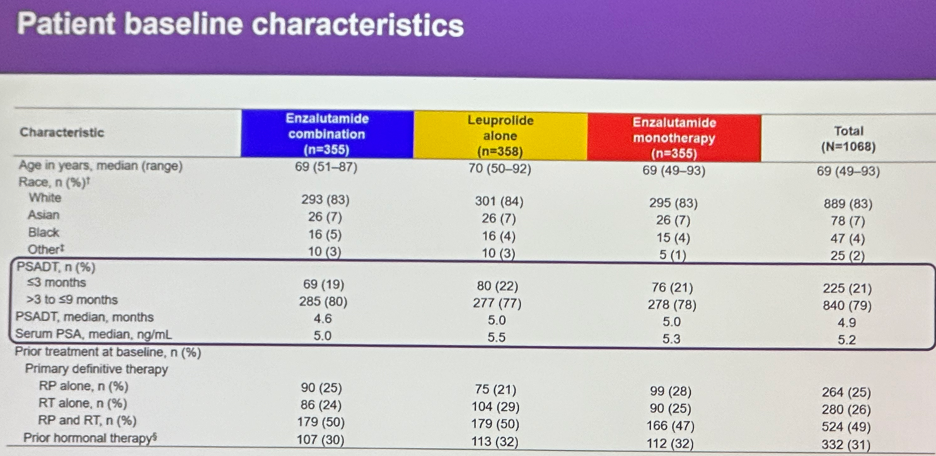
Treatment was suspended at week 37 based on PSA response in 91% of patients in the enzalutamide combination arm, 68% in the leuprolide alone arm, and 86% in the enzalutamide monotherapy arm.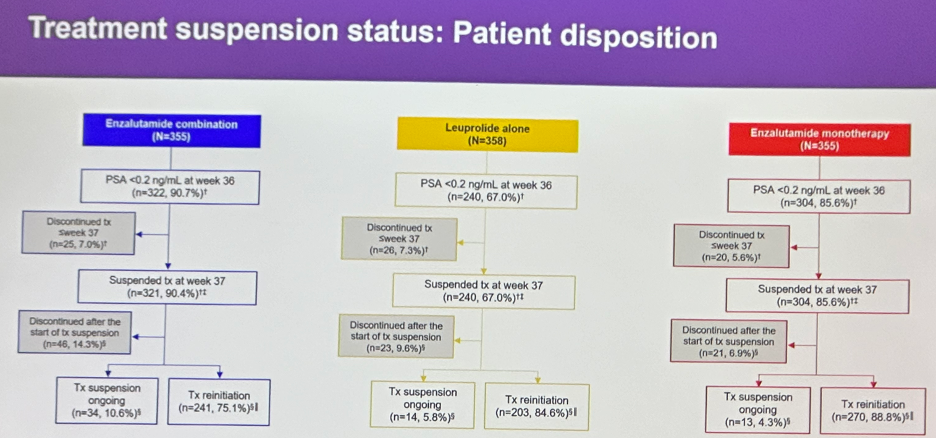
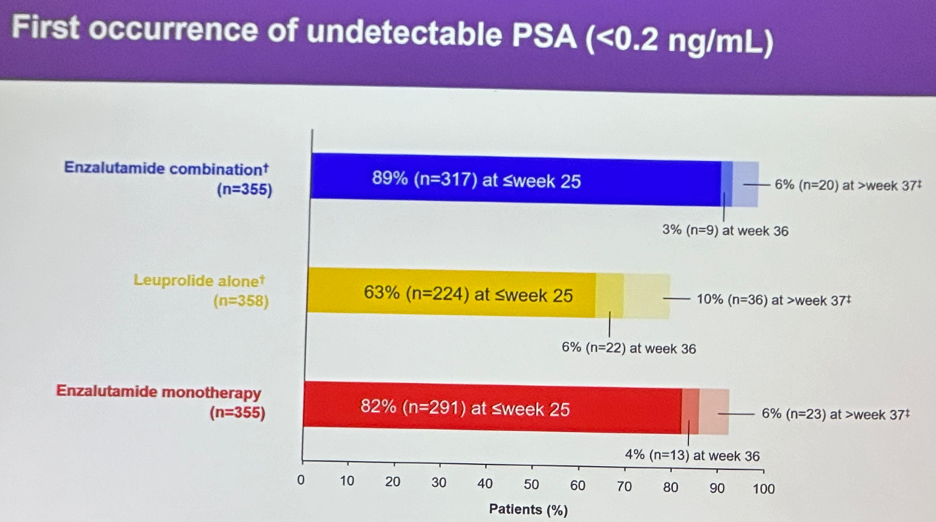
The first occurrence of an undetectable PSA (<0.2 ng/ml) was observed by week 25 in:
- Enzalutamide combination: 89% of patients
- Leuprolide alone: 63%
- Enzalutamide monotherapy: 82%
Among patients who suspended treatment based on an undetectable PSA level, treatment was reinitiated in 75.1%, 84%, and 89% of patients in the enzalutamide combination, leuprolide, and enzalutamide monotherapy arms, respectively.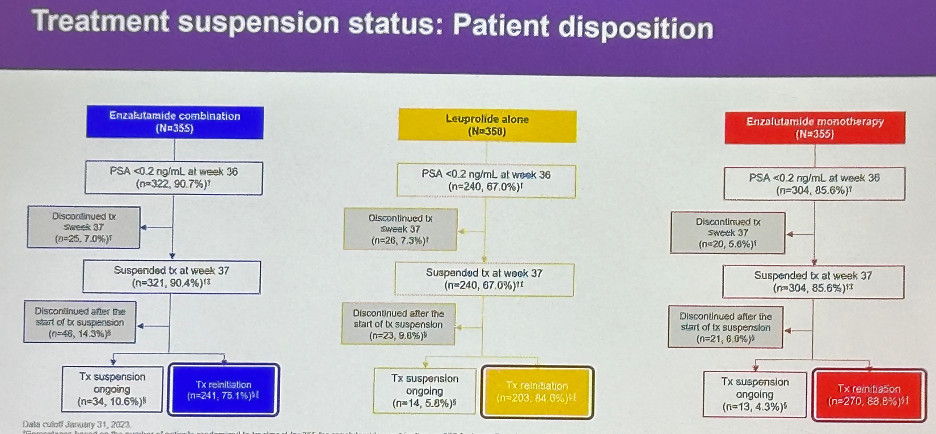
Following this treatment re-initiation, 96% of patients in the enzalutamide combination arm achieved an undetectable PSA. Conversely, 73% and 90% of patients in the leuprolide alone and enzalutamide monotherapy arms reinitiating therapy were able to achieve an undetectable PSA.
Among patients who re-achieved an undetectable PSA reading, metastasis-free survival outcomes were generally favorable across all three treatment arms. Conversely, outcomes were significantly worse in those who did not achieve undetectable PSA levels, similar across all three groups, although the sample sizes for this latter subgroup were noticeably smaller (e.g., n=10 in the enzalutamide combination group).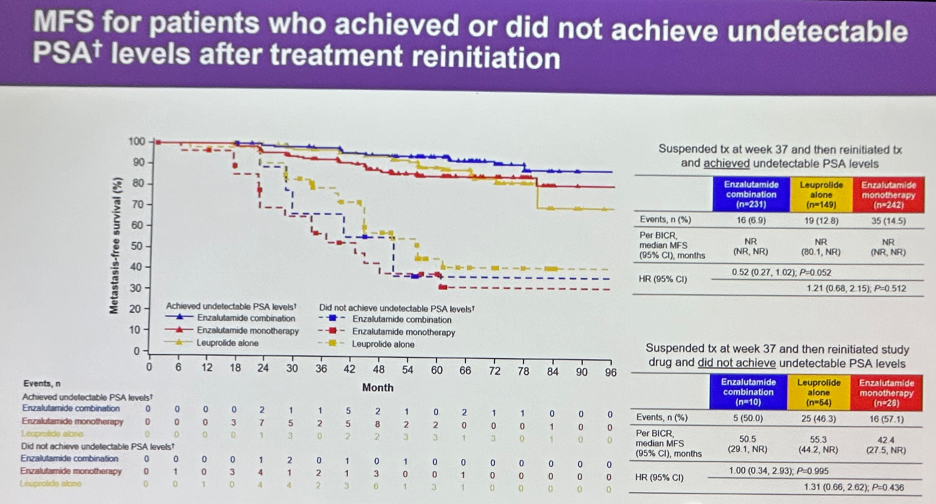
Dr. Freedland concluded as follows:
- In patients with high-risk biochemical recurrence, more patients treated with enzalutamide (both as combination and monotherapy) versus leuprolide alone achieved:
- Undetectable PSA levels at week 36 and subsequent treatment suspension at week 37
- Undetectable PSA levels earlier (week ≤25)
- Undetectable PSA levels after treatment re-initiation
- Due to durable undetectable PSA levels (<0.2 ng/mL), more patients had treatment suspension ongoing in the enzalutamide combination group versus leuprolide alone after a median of 4 years and 11 months.
- Independent of treatment type, achievement of undetectable PSA levels after treatment re-initiation is associated with improved metastasis-free survival
Presented by: Stephen Freedland, MD, Professor, Department of Urology, Cedars Sinai Hospital, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024
References:- FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence.
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Freedland SJ, Gleave M, De Giorgi, et al. Enzalutamide and Quality of Life in Biochemically Recurrent Prostate Cancer. NEJM Evidence. 2023;2(12).


