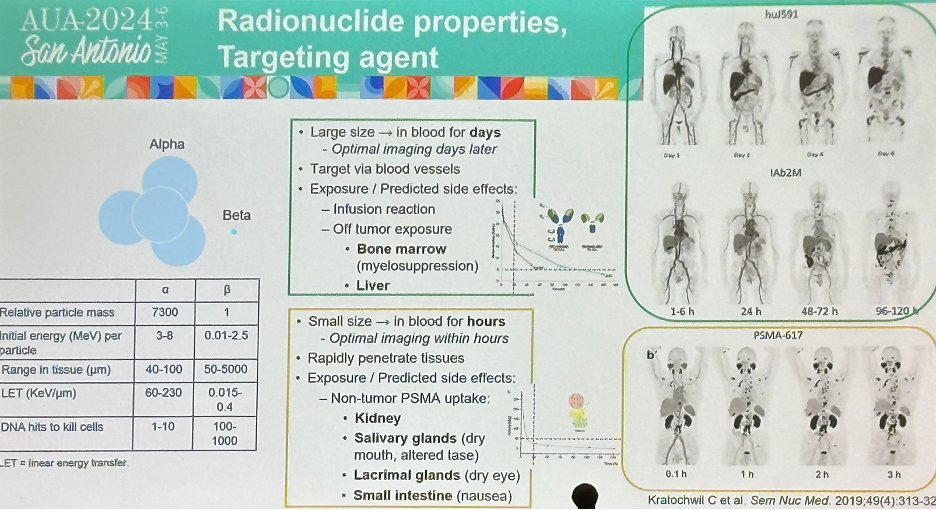
Antibodies and small molecule ligands target PSMA with different kinetics and biodistribution, with certain sites of PSMA expression such as salivary/lacrimal glands, kidneys, and small bowel less accessible to large antibodies. Alpha emitters such as 225Ac have high potency, but short range. They are relatively large in size (7,300-fold larger than beta emitters) and remain present in the blood for days, whereby they subsequently exert their targeted tissue effect via bloodstream distribution.
Dr. Tagawa and colleagues have previously presented the results of a phase 1 trial evaluating single-dose 225Ac-J591. This trial included men with progressive mCRPC following at ≥1 potent androgen receptor pathway inhibitor ([ARPI]; i.e., abiraterone or enzalutamide) and chemotherapy (or unfit/refuse chemotherapy) without a limit on the number of prior therapies. Dose-escalation was in single-subjects x4 followed by 3+3 with a single infusion of 225Ac-J591 (13.3 KBq/kg with planned escalation up to 93.3 KBq/kg).
There were 32 men treated with a single dose of 225Ac-J591 on 7 dose levels with expansion at the highest dose level (n = 16). One of six patients in cohort 6 (80 KBq/kg) had dose-limiting toxicity (grade 4 anemia and thrombocytopenia) with 0 of 6 patients at the highest dose level (93.3 KBq/Kg) and thus this dose was expanded. No minimum tolerated dose was achieved, and the recommended phase 2 dose was set at 93.3 KBq/Kg. With regards to efficacy outcomes, 69% of patients experienced any PSA decline, with 47% achieving a >50% PSA decline, which was similar irrespective of whether patients received prior 177Lu-PSMA therapy.

What is the rationale for this phase 1 dose escalation trial of multiple and fractionated-dose 225Ac-J591 (PSMA-targeted alpha radionuclide)? Fractionated dosing allows for maximizing dose intensity while limiting resistance. This has proven successful with 177Lu-J591 (now phase 3: NCT04876651) and 177Lu-PSMA-617 (now phase 3: NCT06320067). This regimen involves a single fractionated cycle administered on days 1 and 15. Multiple cycles is the ‘typical’ empiric dosing of radionuclides, allowing for potential combining of 225Ac-J591 with other agents with potentially synergistic mechanisms of action.
The eligibility criteria were as follows:
- Progressive mCRPC after an androgen receptor pathway inhibitor and taxane chemotherapy (or unfit/refused).
- ECOG performance status 0–2
- Intact organ function
- 68Ga-PSMA-11 PET/CT demonstrating ≥1 lesion with uptake > liver
- An amendment was introduced allowing for patients with prior 177Lu-PSMA exposure (separate, ongoing cohort which excludes those that received prior 223Ra).
The study endpoints were as follows:
- Primary
- Dose-limiting toxicity
- Recommended phase II dose
- Secondary
- PSA50 response
- Circulating tumor cells count changes
- Progression-free survival
- Overall survival
- PSMA PET results
- Safety
- Patient-reported outcomes
- Exploratory
- Genomics
- Immune assays
- Microbiome
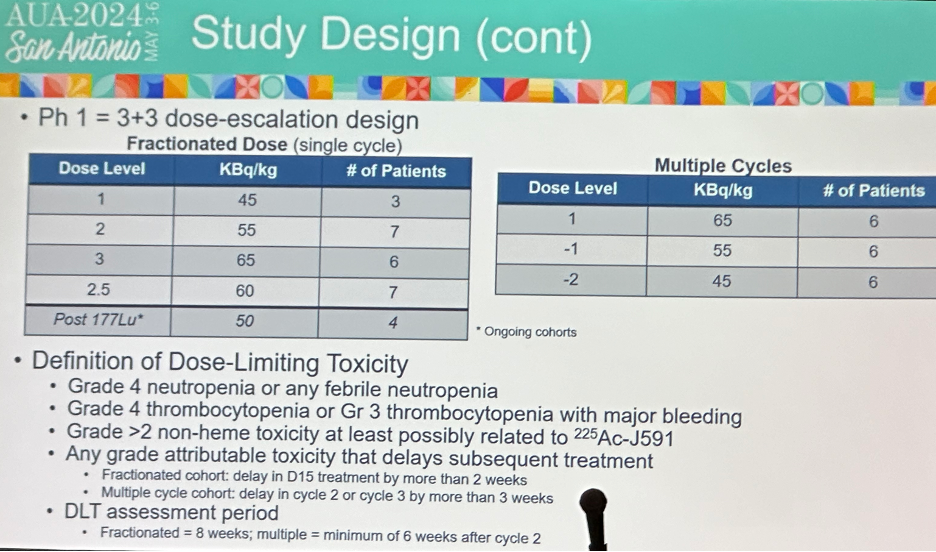
The baseline patient characteristics are summarized below. 18 patients were treated in the multiple cycles cohort (6 per level at 45, 55, 65 Kbq/kg/dose), and 27 in the fractionated dose cohort (45-65 KBq/kg/dose). The median age was 72 years (range: 51–95) and median PSA was 48.8 ng/ml (range: 0.67–2171.17). 53% of patients had CALGB high-risk disease, whereas 35% had intermediate-risk disease. Bone, lymph node, lung, and liver metastases were present in 85%, 68%, 18%, and 18% of patients, respectively. With regards to prior therapy received, 58% had received ≥2 prior ARPIs, 80% ≥1 chemotherapy, 40% sipuleucel-T, 15% 223Ra, and 10% 177Lu-PSMA.

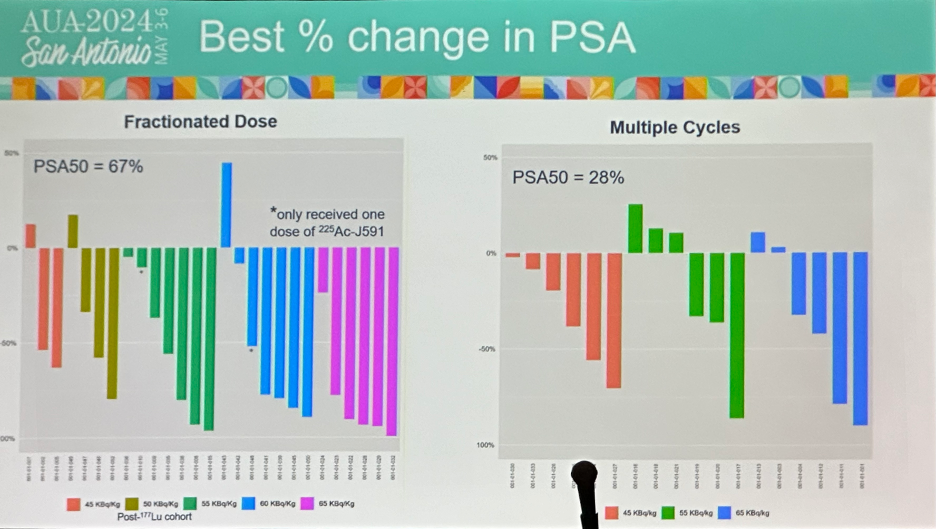
In the multiple cycles cohort, 72% of patients had any PSA decline and 28% achieved a PSA50 response. In the fractionated dose cohort, 95% had any PSA decline and 71% achieved a PSA50 response.
In the fractionated regimen cohort, 3 dose limiting toxicities were observed in (2 in cohort 3 at 65 KBq/kg and 1 in cohort 2.5 at 60 KBq/kg). The recommended phase II dose was determined to be 60 KBq/kg on D1 and D15 (total 120 KBq/kg).
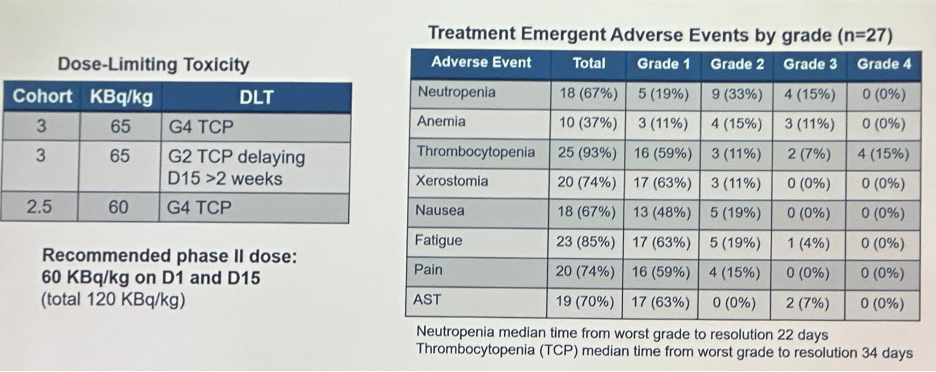
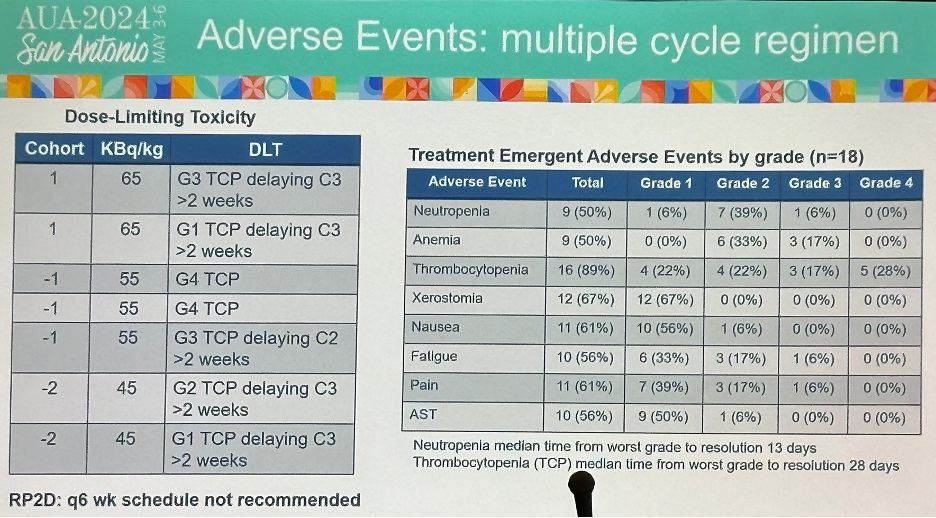
Conversely, in the multiple cycle regimen, 7 dose limiting toxicities were observed, as summarized below. There were 9 grade 3 events and 1 grade 4 event experienced (thrombocytopenia). With regards to the recommended phase 2 dose, the every 6 week dosing schedule was not recommended.
Dr. Tagawa concluded his presentation as follows:
- Fractionated (dose-intense) treatment with 225Ac-J591 appears to be feasible
- Like with 177Lu-J591 (now TLX591), we are able to administer higher cumulative radioactivity in single cycle with fractionation
- 120 KBq/kg (versus 93.3 KBq/kg)
- Transition to phase 2 in 177Lu-PSMA-naïve group
- Phase 1 is ongoing for the post 177Lu-PSMA group
- Licensed to Convergent Therapeutics = CONV01-a
- Developing this regimen in both 177Lu-exposed and naïve
- Multiple dose cycles at 6-week intervals not feasible beyond cycle 2
- But 8-week interval appears feasible even in combination with 177Lu-PSMA small molecules
- Like with 177Lu-J591 (now TLX591), we are able to administer higher cumulative radioactivity in single cycle with fractionation
Presented by: Michael Sun, MD, Hematology-Oncology Fellow, Department of Medicine, Weill Cornell Medicine, New York. NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


