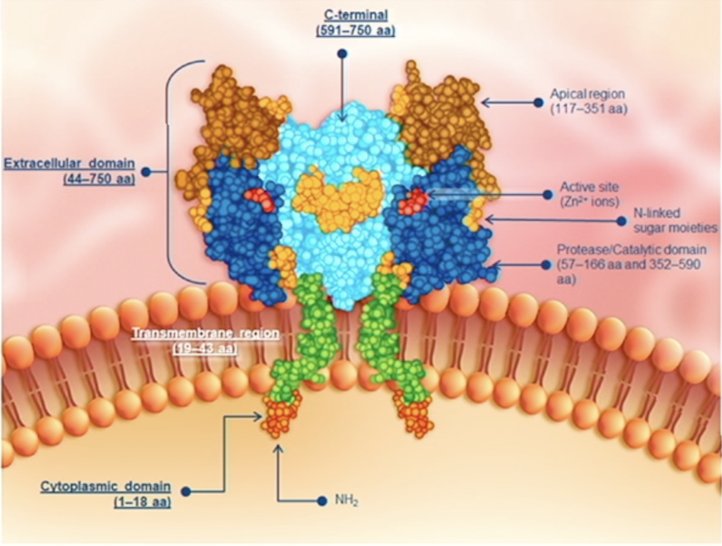
(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a plenary session, and a presentation by Dr. Xiao Wei discussing results from PSMAfore assessing the efficacy of 177Lu-PSMA-617 versus ARPI change in taxane naïve patients with metastatic castration resistant prostate cancer (mCRPC) stratified by pre-randomization ARPI. PSMA is a transmembrane glycoprotein with carboxypeptidase activity that is highly expressed in prostate cancer, including metastatic disease. It has relatively restricted normal expression (ie. salivary and lacrimal glands) and is a target for PET imaging:
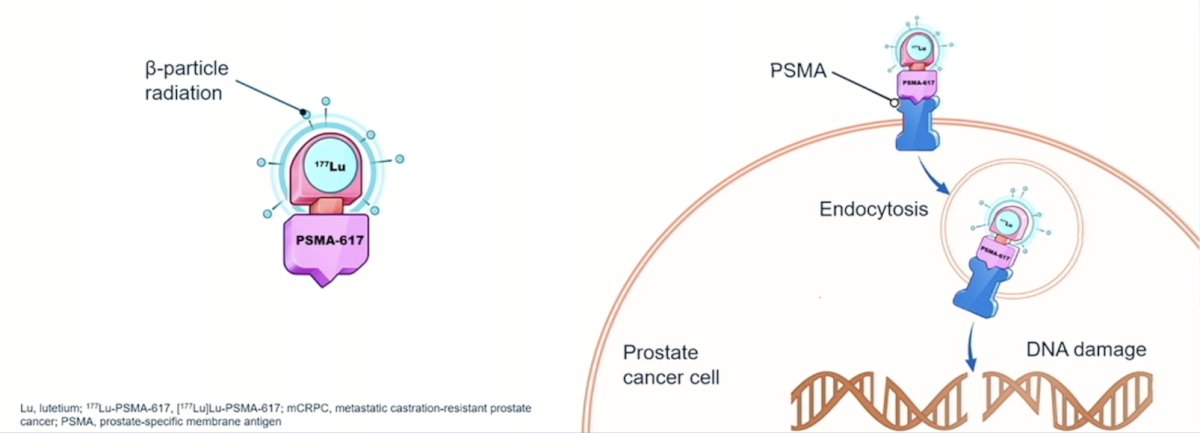
177Lu-PSMA-617 is a targeted radioligand therapy for PSMA-positive mCRPC with the following mechanism of action:
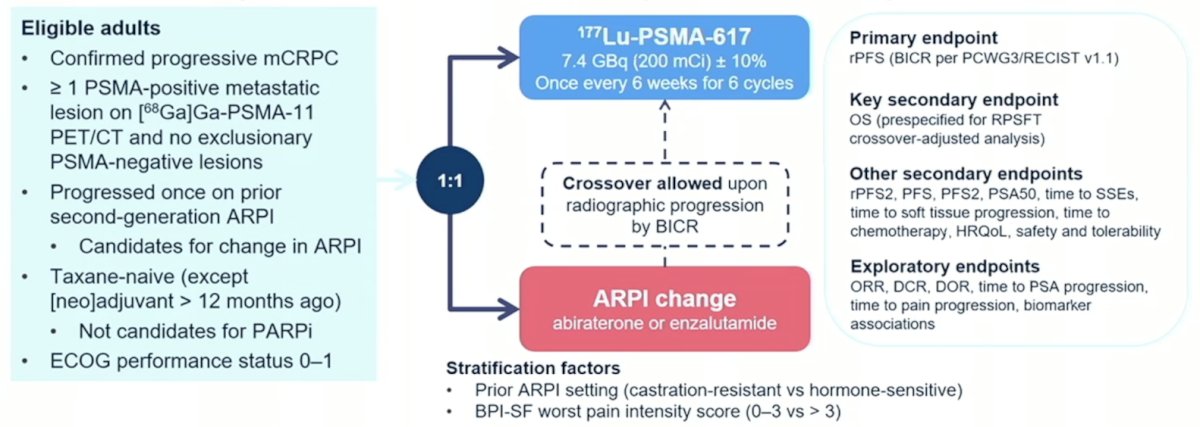
Eligible adults for PSMAfore had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and patients with prior systemic radiotherapy (<6 months ago), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant >12 months ago) were ineligible. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows:
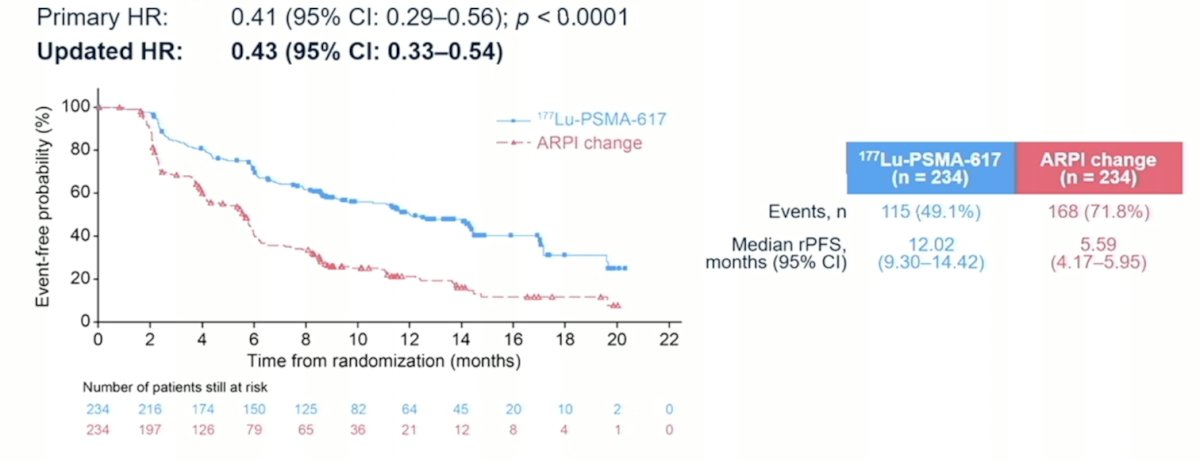
The first interim analysis of PSMAfore was presented at ESMO 2023 by Dr. Oliver Sartor, and the presentation at AUA 2024 represented the second interim analysis with a data cutoff of June 21, 2023. The PSMAfore primary endpoint was radiographic progression free survival, with an updated hazard ratio of 0.43 (95% CI 0.33-0.54) favoring the 177Lu-PSMA-617 arm:
The confirmed PSA50 response was 57.6% for 177Lu-PSMA-617 and 20.4% for the ARPI change arm:
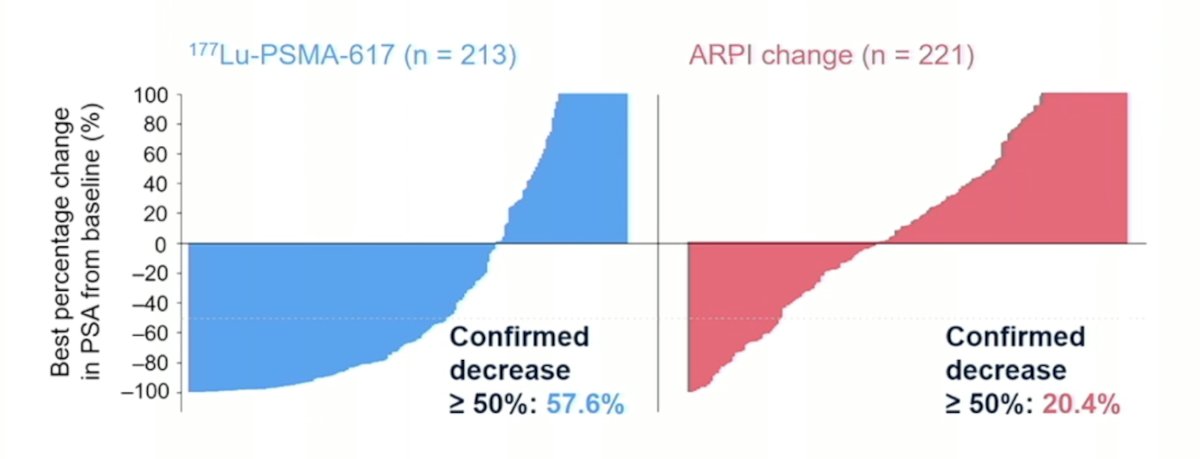
Objective response rate also favored 177Lu-PSMA-617, with a complete response rate of 21.1% versus 2.7%:
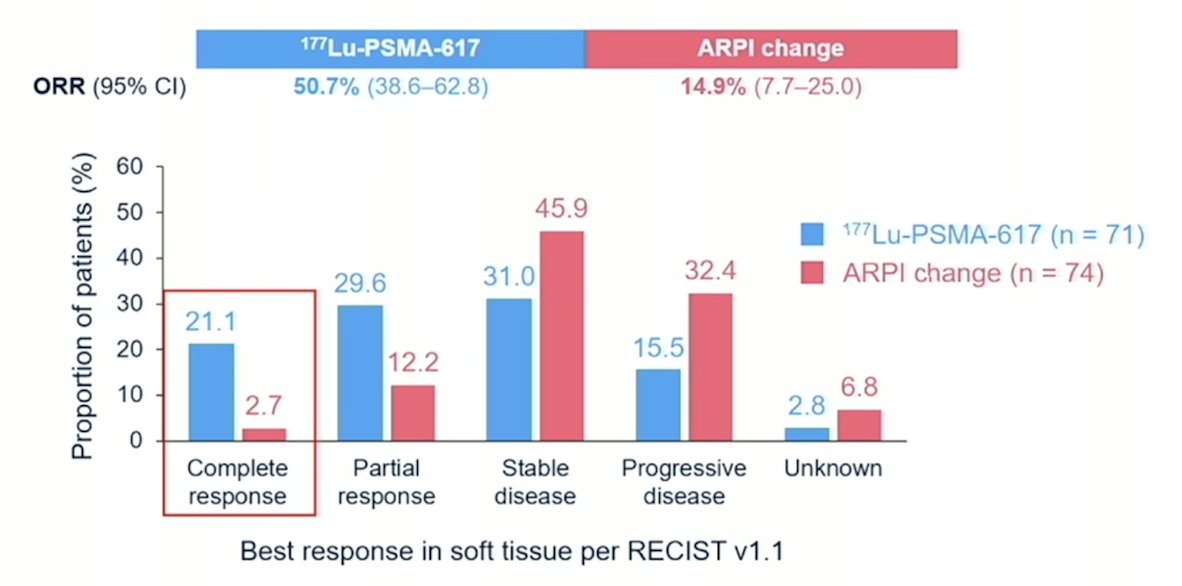
In this post hoc analysis of PSMAfore, Dr. Wei and colleagues assessed efficacy outcomes by pre-randomization ARPI treatment. She notes that in the 177Lu-PSMA-617 arm, 50.9% of patients had prior abiraterone and 40.2% had prior enzalutamide, compared to the ARPI arm where 55.6% of patients had prior abiraterone and 35.9% had prior enzalutamide. The baseline characteristics by arm and stratified by prior abiraterone vs enzalutamide were well balanced:
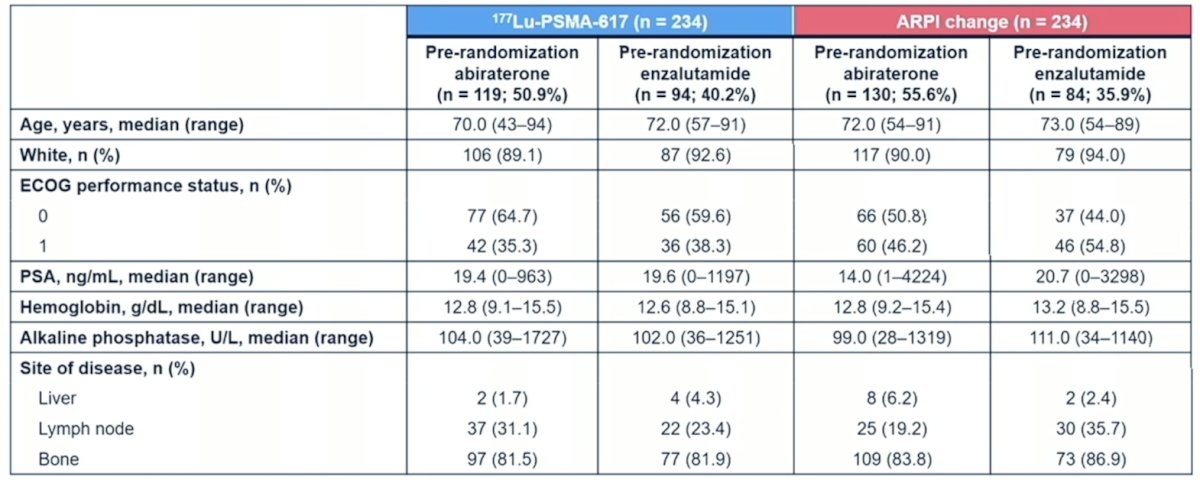
Among patients that underwent pre-randomization abiraterone, 177Lu-PSMA-617 improved radiographic progression free survival versus ARPI change (HR 0.47, 95% CI 0.33-0.66). Similar findings were noted in patients who underwent pre-randomization enzalutamide (HR 0.35, 95% CI 0.24-0.52):
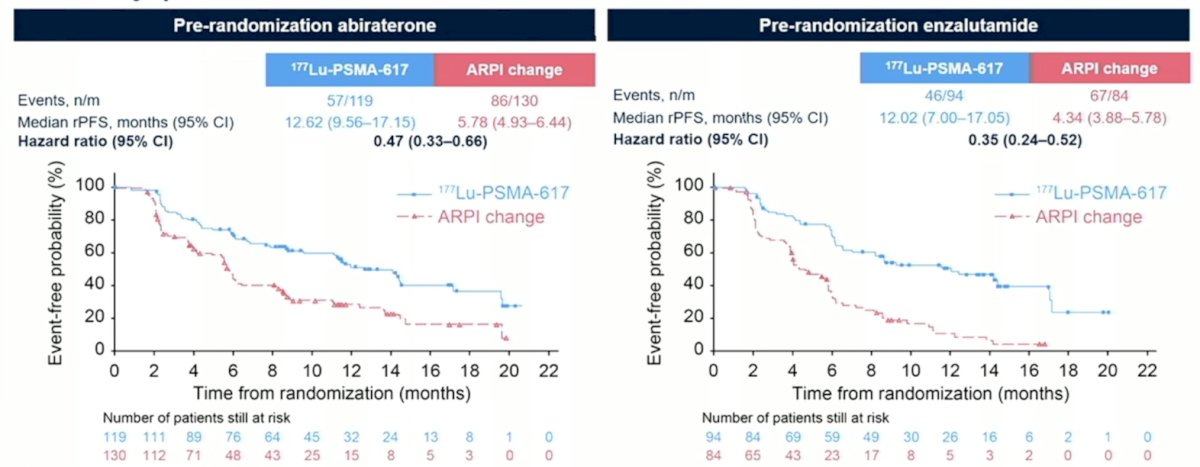
PSA50 responses also favored 177Lu-PSMA-617 in both pre-randomization patients treated with abiraterone or enzalutamide:
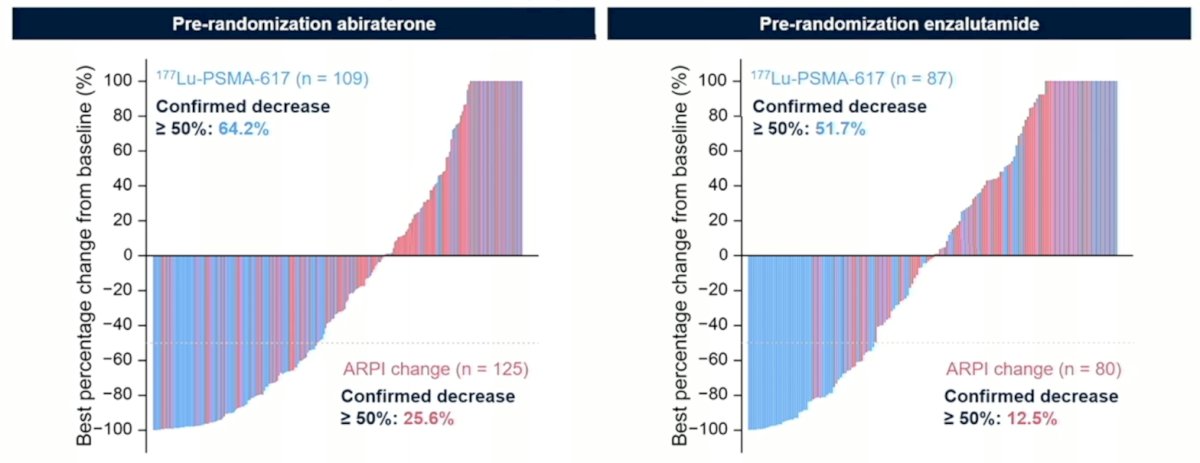
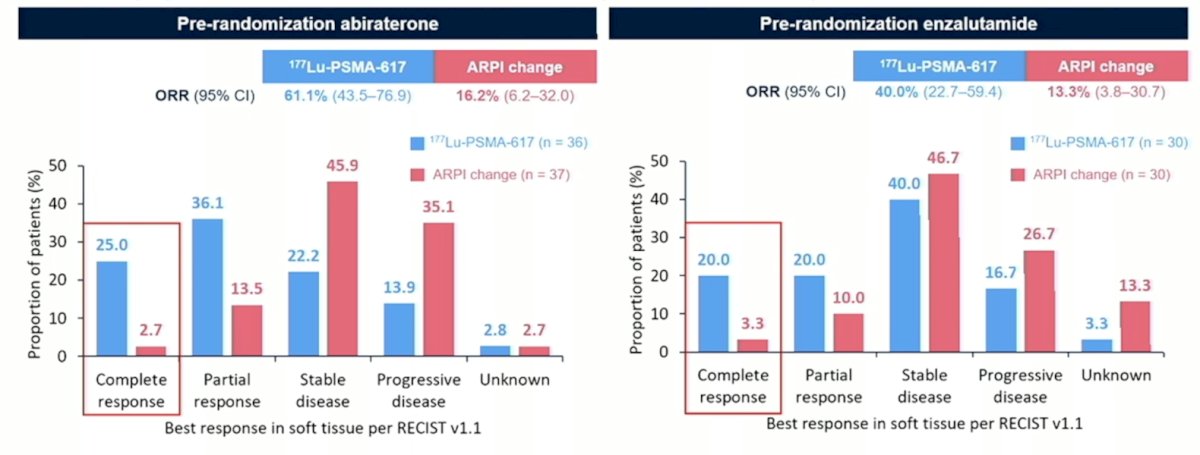
Finally, the complete response rate for 177Lu-PSMA-617 in patients receiving pre-randomization abiraterone was 25.0% vs 2.7% for ARPI change, and for patients receiving pre-randomization enzalutamide was 20.0% vs 3.3% for ARPI change:
Dr. Wei concluded her presentation by discussing results from PSMAfore assessing the efficacy of 177Lu-PSMA-617 versus ARPI change in taxane naïve patients with mCRPC stratified by pre-randomization ARPI with the following take-home messages:
- Updated results of the PSMAfore study at the second interim overall survival analysis showed that 177Lu-PSMA-617 prolonged radiographic progression free survival versus ARPI change irrespective of pre-randomization ARPI in taxane-naïve patients with PSMA positive mCRPC
- Improvements in PSA50 and objective response rate also favored 177Lu-PSMA-617 versus ARPI change, irrespective of pre-randomization ARPI
- Improvements in outcomes favored patients receiving pre-randomization abiraterone versus pre-randomization enzalutamide in both study arms
- These data support the consideration of 177Lu-PSMA-617 as a new standard treatment approach for this highly prevalent patient population of taxane naïve mCRPC
Presented by: Xiao Wei, MD, MAS, Dana Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


