(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a plenary session, and a presentation by Dr. Jason Hafron discussing results from Apa-RP, a multicenter, open-label, single arm trial assessing apalutamide and ADT for the treatment of high-risk localized prostate cancer following radical prostatectomy. In 2024, it is estimated there will be nearly 300,000 new cases of prostate cancer in the US, and ~15% will be diagnosed as having high-risk disease. Approximately, 45–65% of patients with high-risk disease experience recurrence within five years of undergoing radical prostatectomy. Apalutamide is a selective, androgen receptor inhibitor approved for patients with nonmetastatic castration-resistant prostate cancer and metastatic castration-sensitive prostate cancer. Currently, apalutamide is being investigated in two registrational trials in patients with high-risk localized prostate cancer receiving a prostatectomy (PROTEUS; NCT03767244) or radiation therapy (ATLAS; NCT02531516).
Apa-RP is a phase 2 study that investigated apalutamide plus ADT adjuvant to radical prostatectomy in patients with high-risk localized prostate cancer. Key inclusion criteria were high risk localized prostate cancer with a post-radical prostatectomy PSA <= 0.20 ng/mL. The trial design for Apa-RP is as follows:
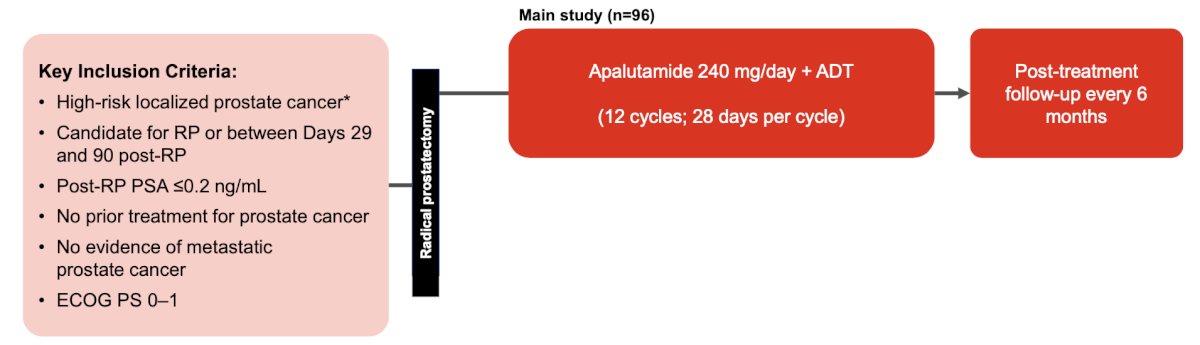
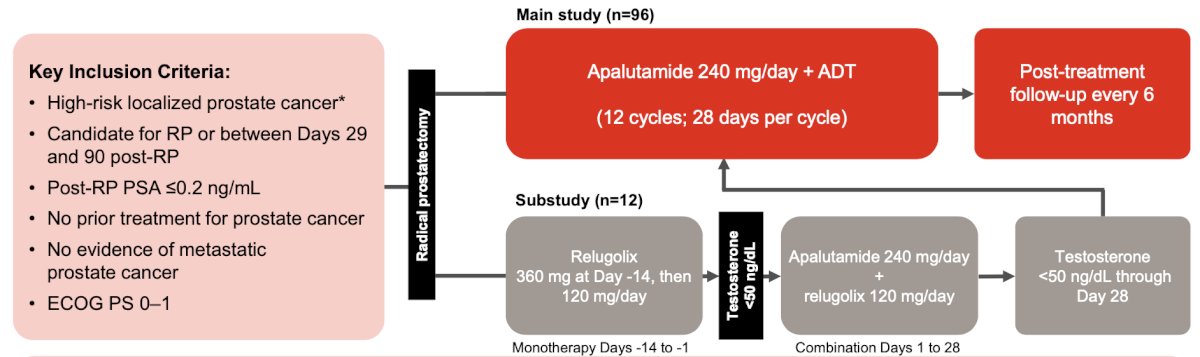
The primary endpoint was biochemical recurrence free survival at 24 months, and the secondary endpoints were biochemical free survival at 12 months and serum testosterone recovery to >= 150 ng/dL at 18 and 24 months. An exploratory endpoint was an unconfirmed biochemical recurrence, defined as a PSA >= 0.2 ng/dL and no subsequent PSA value during the trial. In a study subset, 12 patients received relugolix who were also incorporated into the trial:
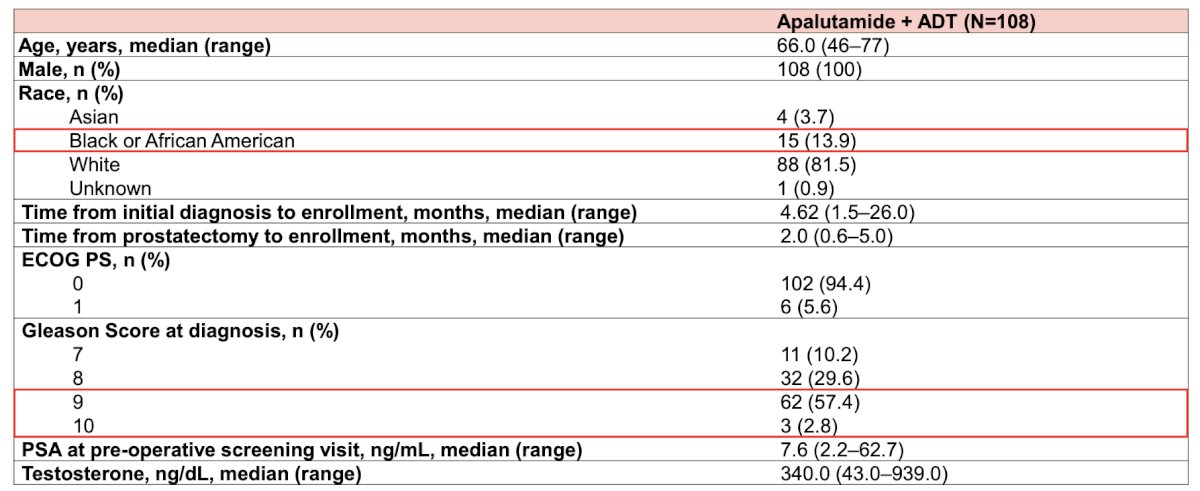
For the primary endpoint, the statistical hypothesis was a 10% absolute increase compared with a historical control being a 76% biochemical recurrence-free rate at 24 months based on data from Martini et al.1 At a one sided alpha of 0.05 and 80% power, the minimum number of patients needed to be enrolled would by 94, with a goal to enroll 96 patients to account for patient attrition. There were 108 patients enrolled from August 2020 to October 2023 from 27 community urology sites. The median age was 66.0 years (range: 46-77), with 81.5% white and 13.9% Black/African American, and ~60% of patients with Gleason Score 9-10. The median preoperative PSA was 7.6 ng/mL (range: 2.2-62.7):
There were no patients that had a confirmed biochemical recurrence at 2+ years following radical prostatectomy, with a confirmed biochemical recurrence-free rate at 24 months of 100% (90% CI 93.0 – 100.0):
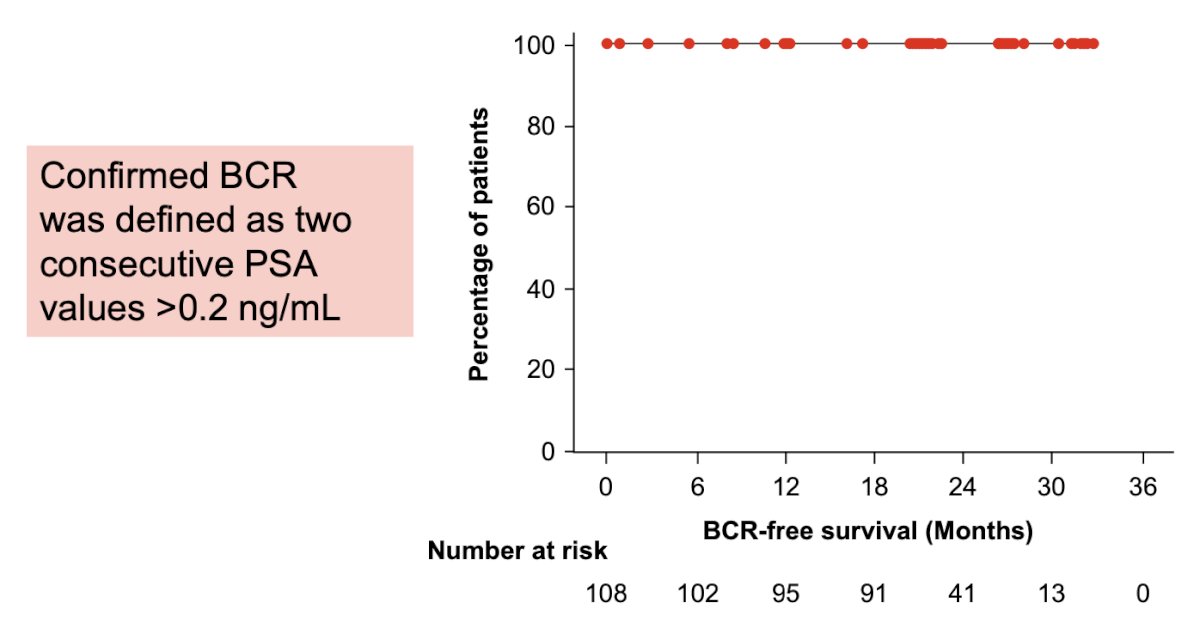
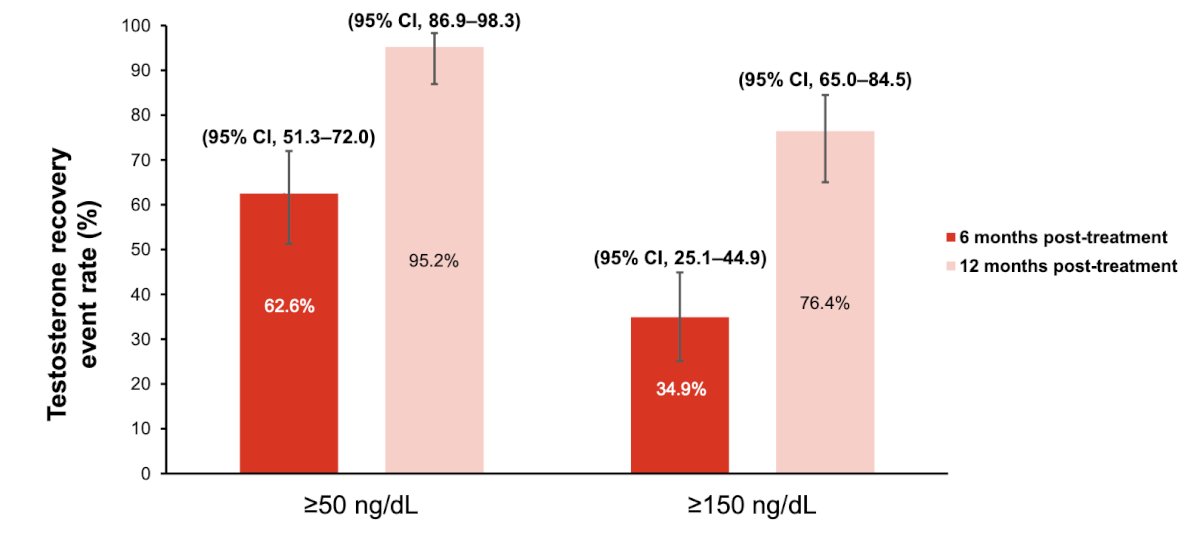
Two patients had an unconfirmed biochemical recurrence at 2+ years following radical prostatectomy (patient 1, PSA 0.39 ng/mL at 24 months; patient 2, PSA 0.22 ng/mL at 30 months), with an unconfirmed biochemical recurrence free rate at 24 months of 98.4% (90% CI 92.2-99.7). There were 76% of patients that had a testosterone recovery to 150 ng/dL at 12 months following treatment completion:
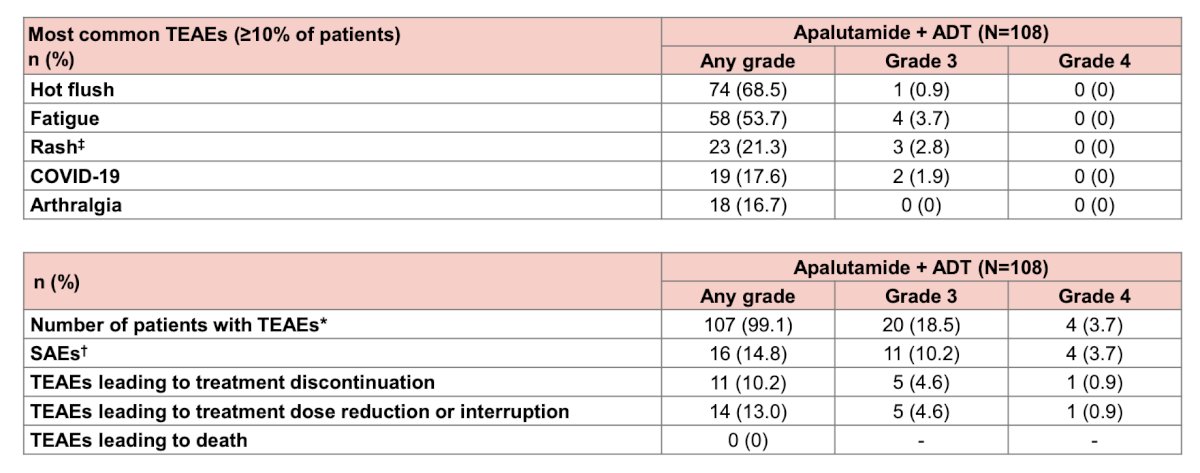
The safety profile of apalutamide + ADT was consistent with previous reports:
Dr. Hafron concluded his presentation by discussing the results from Apa-RP with the following take-home messages:
- Biochemical recurrence-free rate was 100% at 24 months, with two patients having unconfirmed biochemical recurrence
- Serum testosterone recovery rates to ≥150 ng/dL were 34.9% at 6 months post-treatment and 76.4% at 12 months post-treatment
- Per FDA guidance, the enrolled population was reflective of the US population (~15% of patients enrolled in Apa-RP were Black)
- The safety profile of apalutamide + ADT was consistent with what has been reported previously
- Treatment intensification with 12 cycles of apalutamide + ADT could become an option for patients with high-risk localized prostate cancer who have undergone radical prostatectomy
Presented by: Jason Hafron, MD, Michigan Institute of Urology, West Bloomfield, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Martini A, Gandaglia G, Karnes RJ, et al. Defining the most informative intermediate clinical endpoints for predicting overall survival in patients treated with radical prostatectomy for high-risk prostate cancer. Eur Urol Oncol. 2019 Jul;2(4):456-463.


