(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX was host to the advanced prostate cancer moderated poster session. Dr. Ryuma Tanaka presented the results of a retrospective study of patients with metastatic hormone sensitive prostate cancer (mHSPC) and the impact of delayed administration of androgen receptor pathway inhibitors (ARPIs) on oncological outcomes and prognosis.
This study included 621 patients with mHSPC between January 2014 and March 2023, who had been treated either with androgen deprivation therapy (ADT) + complete androgen blockade (CAB) (ADT + nonsteroidal anti-androgen) n= 379 or ADT with upfront ARPIs n=243. Patients were stratified according to the time of ARPI initiation in the ARPI cohort. In the ADT/CAB cohort patients were classified as responder (3 months PSA ≤ 0.2) or non-responder (PSA at 3 months >0.2), (Figure Below).
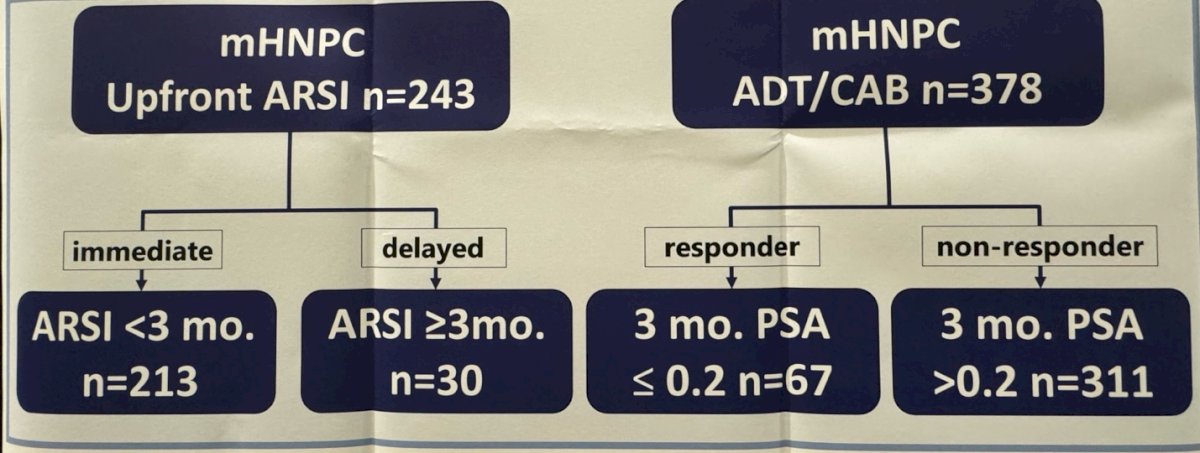
The main outcomes of the study were castration resistant prostate cancer (CRPC) free survival and overall survival (OS). They compared patients who started the ARPIs <3 months (immediate upfront group) and≥3 months (delayed upfront group).
The investigators showed that in the ARPI upfront therapy group, they identified 213 and 30 patients in the immediate and delayed groups, respectively. The median time from ADT initiation to initiation was 0.4 months for the immediate group and 4.6 months for the delayed group.
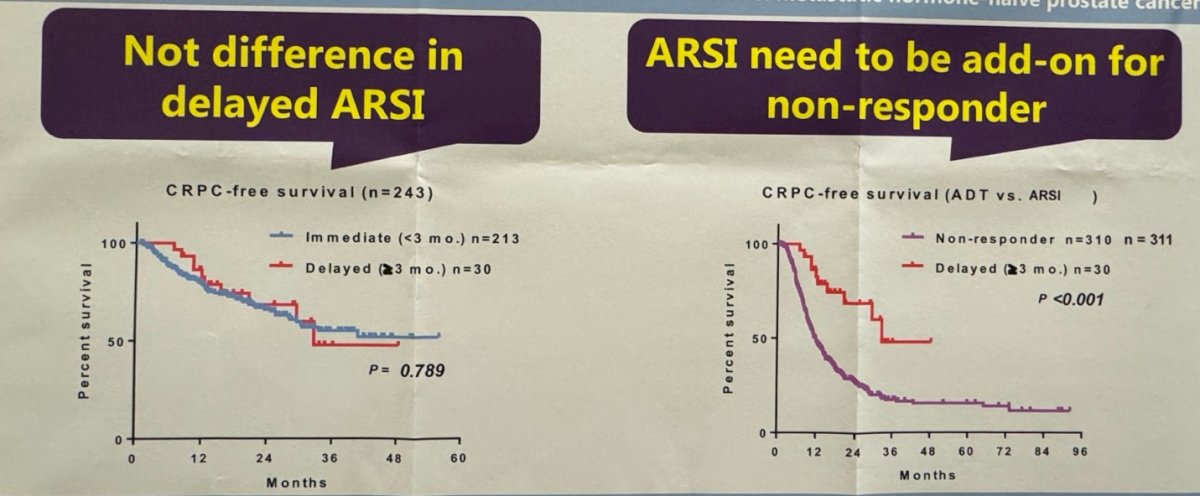
The investigators found no significant differences in terms of CRPC-free survival and OS between the immediate and delayed groups.

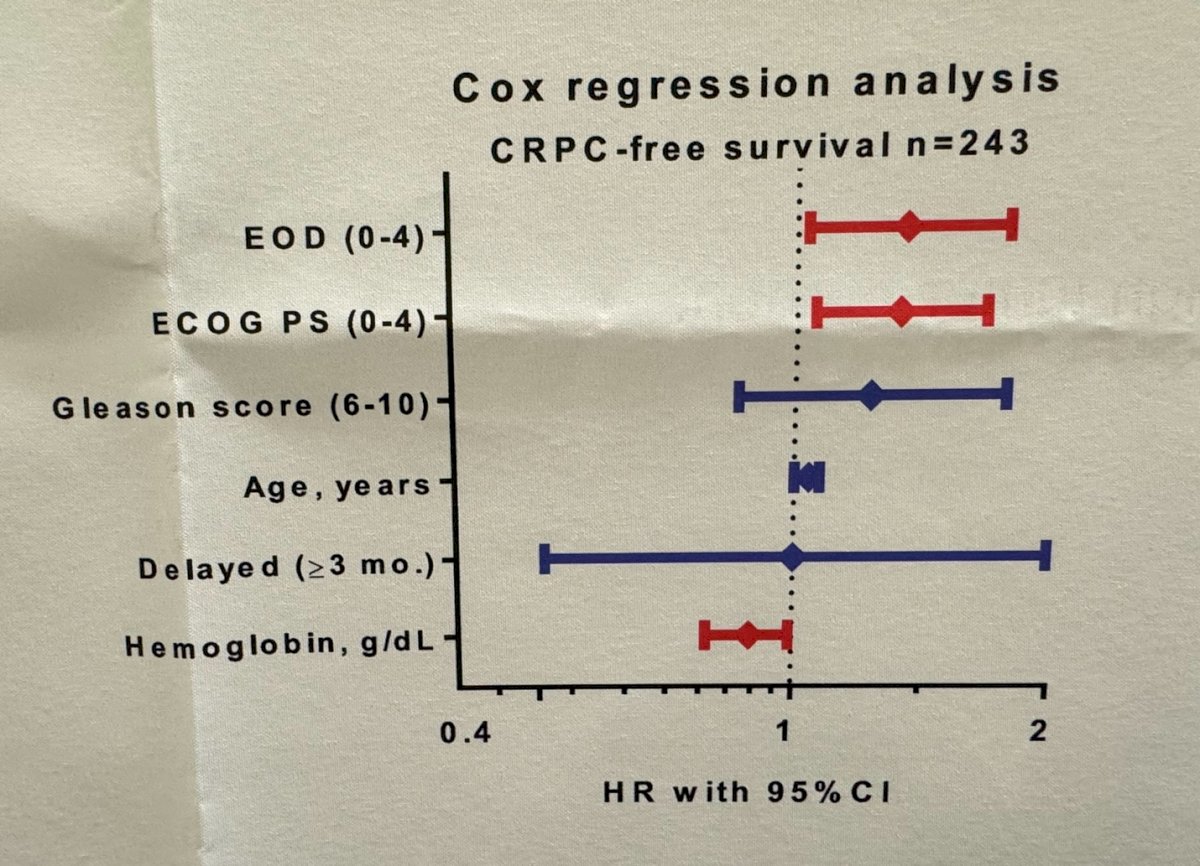
In multivariable Cox regression analysis, they found the volume of bone metastasis and performance status were significantly associated with CRPC-free survival.
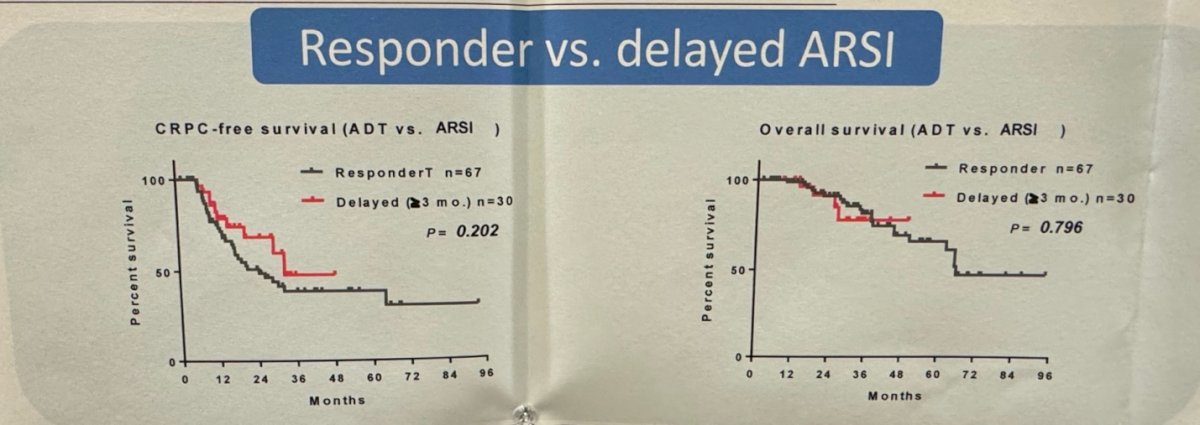
Furthermore, they investigated whether patients who received "vintage" therapy ADT/CAB had a better PSA response compared to the immediate and delayed upfront ARPI groups. They reported that among the ADT/CAB therapy group, only 6 patients (17.9%) were PSA responders (PSA ≤0.2 ng/mL). This response rate was significantly lower compared to the immediate upfront group (31.5%), but similar to the delayed upfront group (16.7%). No significant differences in CRPC-free survival or overall survival were found between patients with PSA who were responders in the ADT/CAB or delayed ARPI groups
There was a significant difference in CRPC-free survival between patients in the delayed ARPI group and non-responders in the ADT/CAB group (p<0.001). They suggested that this difference could have been mitigated by adding the ARPI to the non-responder patients in the ADT+CAB group.
Dr Tanaka concluded the presentation by saying:
- Delayed (≥3 months) administration of ARPIs in patients with de novo mHSPC could be feasible for initial PSA responders on ADT/CAB and may not translate into worse oncological outcomes.
- For patients who are non-responders on initial ADT/CAB, upfront ARPI might be necessary as an add-on therapy, if not started upfront.
Presented by: Ryuma Tanaka, MD, at the Department of Urology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


