(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the advanced prostate cancer moderated poster session. Dr. Neal Shore presented the results of a phase 1 cohort of a phase 1/2 study evaluating ARV-766 which is a second-generation PROteolysis TArgeting Chimera (PROTAC) AR degrader that targets wild-type AR and clinically relevant mutants with the addition of abiraterone for the treatment of novel-hormone-agents (NHA)-naïve metastatic castration-resistant prostate cancer (mCRPC) or metastatic castration sensitive prostate cancer (mCSPC).
Dr. Shore began by explaining the mechanism of action of ARV-766. It's a PROTAC androgen receptor (AR) degrader that forms a trimer complex with the AR and an ubiquitin ligase, triggering ubiquitination and subsequent degradation of the AR by the proteasome.
Preclinical data demonstrating ARV-766's degradation activity against wild-type AR and several other clinically relevant AR mutants showed significant tumor growth inhibition in mouse models of prostate cancer. Abiraterone functions by depleting androgen biosynthesis, and resistance to abiraterone may arise from AR upregulation, AR mutations, or splice variants. There exists a theoretically synergistic relationship between Abiraterone and ARV-766, as the PROTAC AR degrader targets wild-type and mutant AR for degradation enhancing the efficacy of abiraterone when the two drugs are combined.
The phase 1/2 study (NCT05067140) evaluates ARV-766 in three different parts. Parts A and B explored ARV-766 monotherapy in men with mCRPC (target n=150). More specifically:
- Part A (dose escalation): patients who had ≥2 prior therapies (including ≥1 NHA) were given ARV-766 orally once daily in ascending doses to assess safety and tolerability and select recommended phase 2 doses.
- Part B (cohort expansion): patients treated with 1–3 prior NHAs and ≤2 chemotherapy regimens were randomized to receive 100 mg or 300 mg of ARV-766 orally to evaluate the antitumor activity of the recommended phase 2 doses.
- Part C: assess the addition of ARV-766 to abiraterone for the treatment of NHA-naïve mCRPC or mCSPC, which was presented here today at the AUA 2024 meeting.
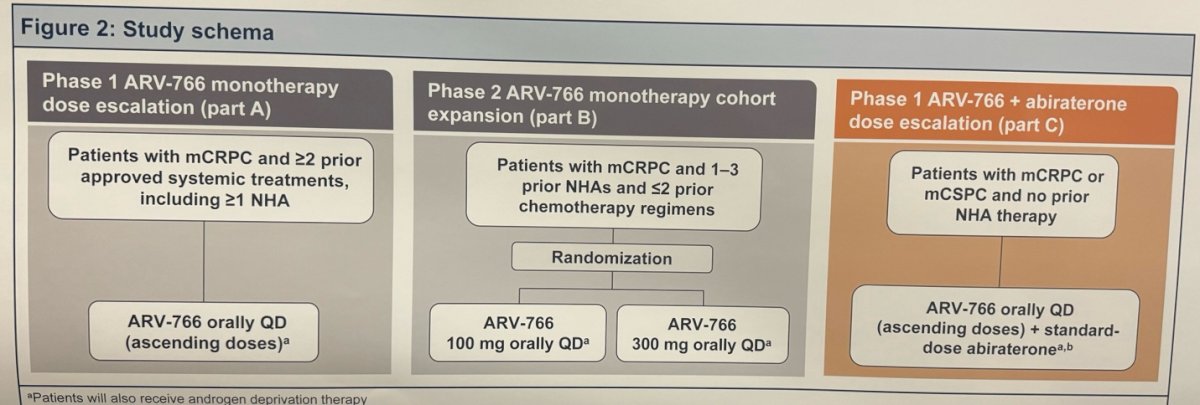
Part C enrolled ≤24 men, aged ≥18 years with histologically, pathologically, or cytologically confirmed mCRPC or mCSPC. Participants were required to have an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, and no prior NHA treatment. The patients will receive escalating doses of ARV-766 (starting at 100 mg QD) with standard-dose abiraterone (1000 mg) to assess drug-drug interaction (DDI) and safety of the combination; additionally, the patients will also receive androgen deprivation therapy (ADT) of choice.
The primary endpoints of part C are: first-cycle dose-limiting toxicities and frequency and severity of adverse events (AEs) and laboratory abnormalities.
The investigators discussed that the safety profile of ARV-766 has been demonstrated in ARV-766 monotherapy (n=84), with the most common treatment-related adverse events (TRAEs) being fatigue (29%) and nausea (14%). A detailed list of TRAEs is provided in the table below: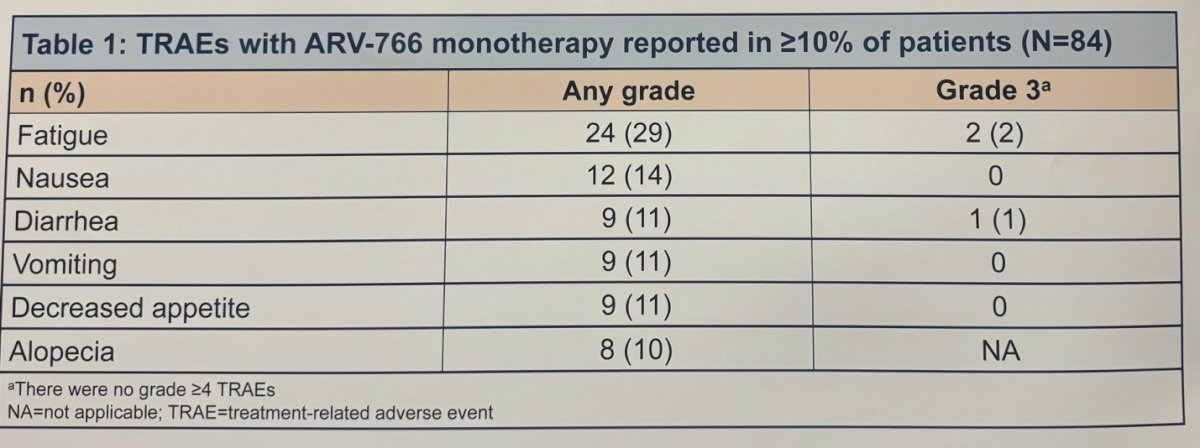
Dr. Shore concluded his presentation by suggesting that combining ARV-766 with Abiraterone may yield promising results in mCRPC and mHSPC due to their potential synergistic relationship. However, further confirmation and follow-up of Part C of this study are still needed.
Presented by: Neal Shore, MD, FACS Director, CPI, Carolina Urologic Research Center, Chief Medical Officer of Surgery and Oncology, GenesisCare USA, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024


