(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the advanced prostate cancer moderated poster session. Dr. Rossella Nicoletti presented the results of the PIONEER study, the European network of excellence for big data in prostate cancer evaluating real-world trends and outcomes for European patients with metastatic hormone-sensitive prostate cancer (mHSPC).
They began by highlighting the increasing evidence since 2015 supporting early treatment intensification for mHSPC patients, showing improved overall survival. However, despite this robust evidence informing guideline updates, the implementation of these recommendations in clinical practice has historically been inadequate. Consequently, the analysis of real-world data becomes crucial in assessing the practical application of such guidelines as well as identifying relevant outcomes and clinical cohorts with unmet clinical needs who could benefit from treatment intensification. This study aimed to delineate the demographics, clinical profiles, treatment modalities, and clinical outcomes of a large multicenter cohort of mHSPC patients utilizing real-world data within the framework of the PIONEER project.
Dr Nicoletti explained they used data from patients with mHSPC across a distributed network of observational databases and divided the patients into two large cohorts:
- Cohort 1: Male patients without prior ADT with mHSPC
- 1.1 Metachronous mHSPC
- 1.2 Synchronous mHSPC
- Cohort 2 The start of ADT as a proxy definition of mHSPC disease.
- 2.1 Metachronous mHSPC
- 2.2 Synchronous mHSPC

This analysis included 94,261 mHSPC patients in cohort 1 and 77,123 in cohort 2. Over half (54%) of the patients were older than 70 years. Specifically in cohort 2, a total of 2,819 patients had metachronous mHSPC or were diagnosed with mHSPC after local/primary treatment, while 55,502 patients had synchronous mHSPC or de novo disease.
Despite clinical practice guidelines recommendations and robust evidence for treatment intensification in mHSPC, most patients are still treated with ADT only. Using real-world data from the PIONEER study, the investigators reported that only 28% of mHSPC patients received combination therapies (ARPI/Docetaxel + ADT). With a median follow-up of 500 days (IQR 398-699 days) in the metachronous setting, 22% of patients discontinued treatment. Below, the graphic shows the treatment patterns of mHSPC using real-world data.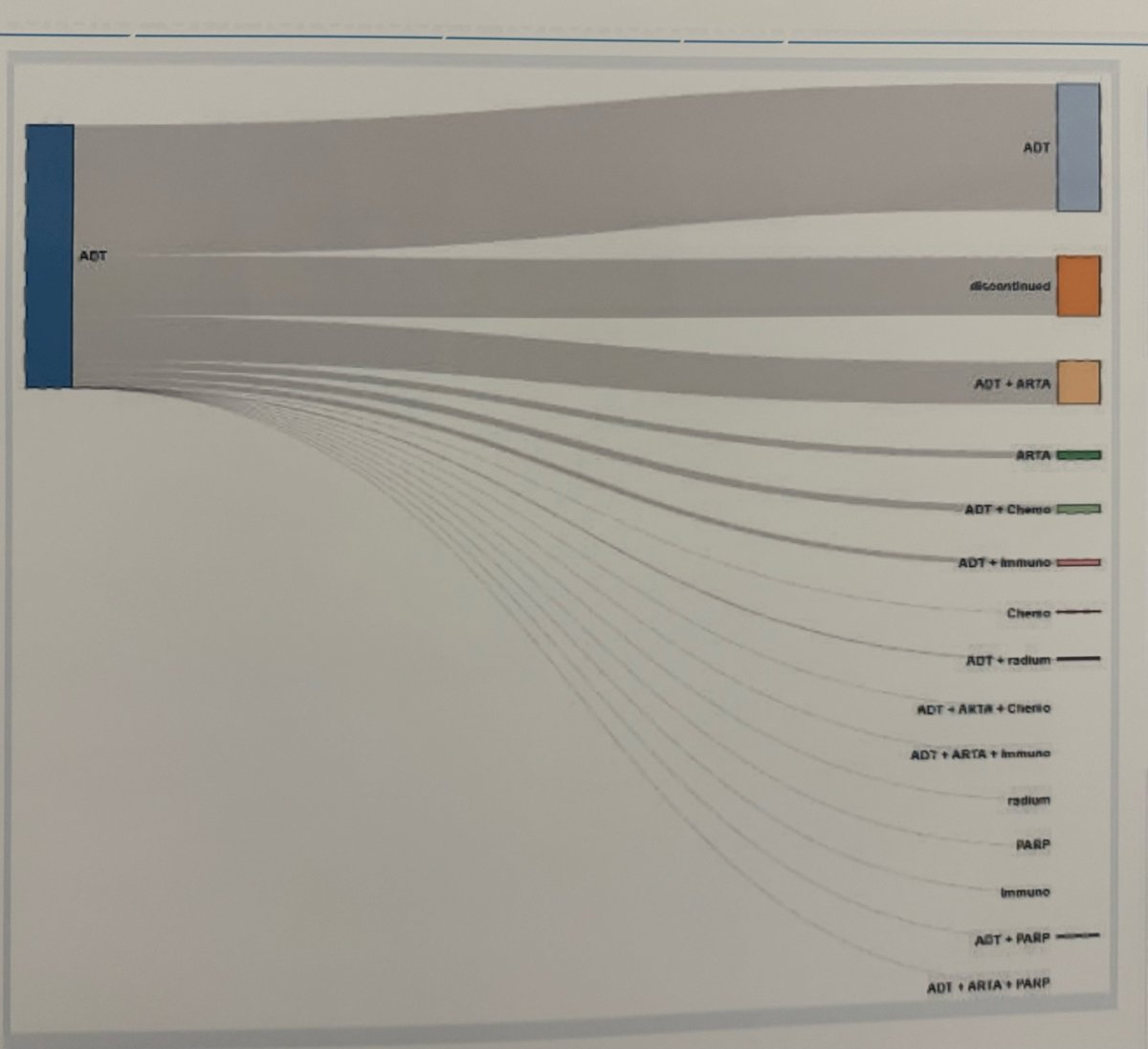
Dr. Nicoletti concluded her presentation by stating that, to date, this is the largest study in Europe reporting real-world data in the mHSPC setting. They found that, according to real-world data, patients are older, with more comorbidities than those in the pivotal clinical trials of mHSPC, and approximately one-third of them do not undergo treatment intensification, which is now the standard of care treatment.
Presented by: Rossella Nicoletti, MD, Urology Resident, Careggi University Hospital in Florence, Italy.
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024


