(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to the International Prostate Forum. Dr. Jeremie Calais presented key updates in radioligand therapy for patients with PSMA-sensitive disease.
The field of prostate cancer theranostics has experienced tremendous growth over the past decade. In 2015, Benesova et al. described one of the earliest experiences with PSMA-targeted Lutetium-177 radioligand therapy, demonstrating biochemical and radiographic responses in patients with advanced prostate cancer.1 This was shortly followed by a report from Kratochwil et al. that similarly demonstrated remission with 177Lu-PSMA in a metastatic prostate cancer patient.2

177Lu-PSMA therapy allows for the delivery of beta radiation over time, inducing clustered DNA damage via ionizing radiation. It has a physical half-life of 6.65 days, with a longer effective half-life owing to its pharmacokinetic profile.

There are two major trials in the metastatic castrate-resistant prostate cancer (mCRPC) space that have led to the regulatory approval of 177Lu-PSMA-617 in the post-taxane and androgen receptor pathway inhibitor (ARPI) setting: VISION and TheraP.3,4
VISION is an international, open-label, phase 3 trial that evaluated 177Lu-PSMA-617 in mCRPC patients previously treated with an androgen receptor pathway inhibitor (ARPI) and 1-2 taxane regimens and who had PSMA-positive 68Ga-PSMA-PET/CT scans. Between June 2018 and October 2019, 831 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus protocol-permitted standard care or standard care alone. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus standard care significantly prolonged, as compared with standard of care, both radiographic progression-free survival (median: 8.7 versus 3.4 months; HR: 0.40, p<0.001) and overall survival (median: 15.3 versus 11.3 months; HR: 0.62; 95% CI: 0.52 to 0.74, p<0.001).
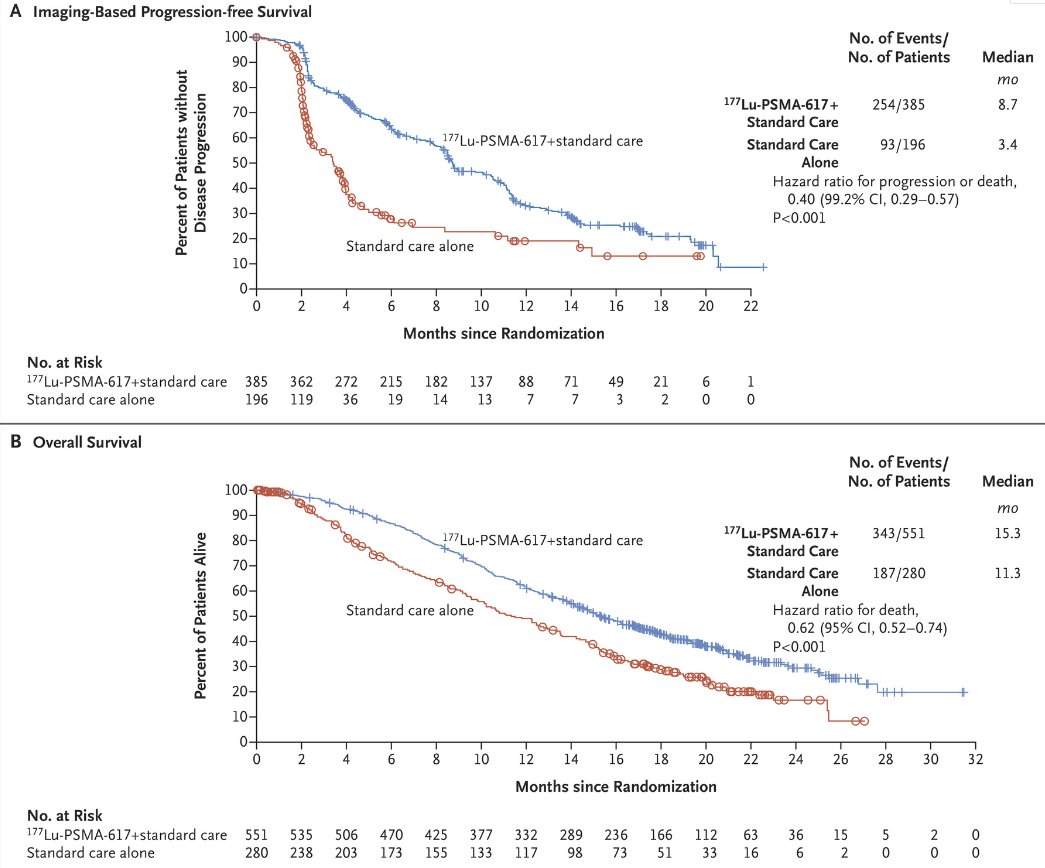
Following the results of this trial, supported by those from TheraP, PLUVICTOTM (177Lu vipivotide tetraxetan) was approved in March 2022 for the treatment of adult patients with PSMA-positive mCRPC who have been previously treated with an ARPI and taxane-based chemotherapy. The current recommended dosage is 7.4 GBq (200 mCi) intravenously every 6 weeks for up to 6 doses or until disease progression, or unacceptable toxicity.

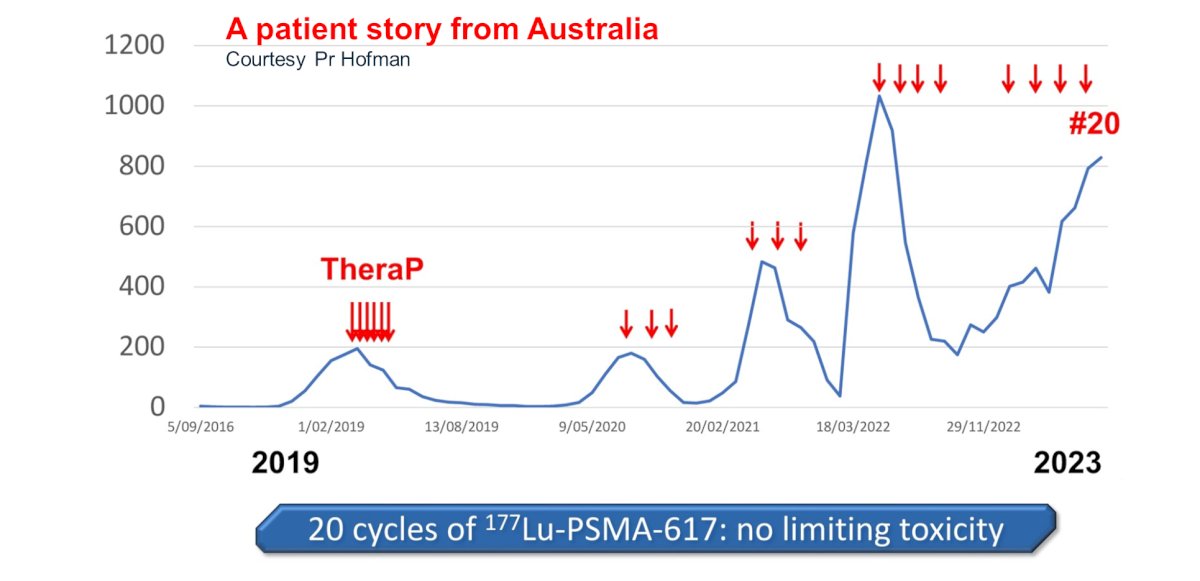
Is there additional value in administering more than 6 cycles? Mader et al. have demonstrated that radioligand therapy is a feasible treatment option in patients with high-volume residual tumor after the completion of standard treatment with six cycles of [177Lu]Lu-PSMA-617.5 Such additional cycles may be administered in either a continuous fashion or as a re-challenge. In a retrospective cohort of 111 patients treated with 177Lu-PSMA, Seifert et al. demonstrated that a 177Lu-PSMA re-challenge is associated with superior PSA50 responses and overall survival outcomes compared to those receiving continuous radioligand therapy. The authors concluded that the response to [177Lu]Lu-PSMA rechallenge demonstrates preserved efficacy of [177Lu]Lu-PSMA after a treatment break.6 The phase 2 RE-LuPSMA (NCT06288113) trial will open for enrollment in 2024 at UCLA.

Further evidence to support >6 cycles and drug holidays can be seen from the TheraP trial, whereby one trial participant has received 20 cycles of 177Lu-PSMA-617 over five distinct periods, with no dose limiting toxicities noted and observed PSA responses with each treatment re-challenge.
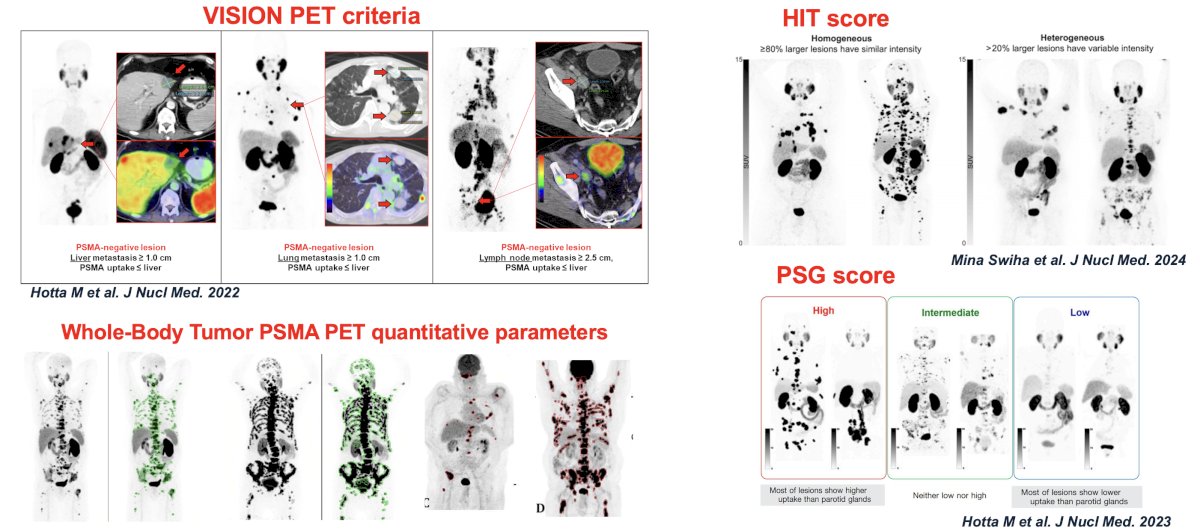
How do we identify non-responders to 177Lu-PSMA using PSMA PET? There have been numerous scores/classification systems identified, including the VISION PET criteria, HIT score, PSG score, and whole-body tumor PSMA PET quantitative parameters.
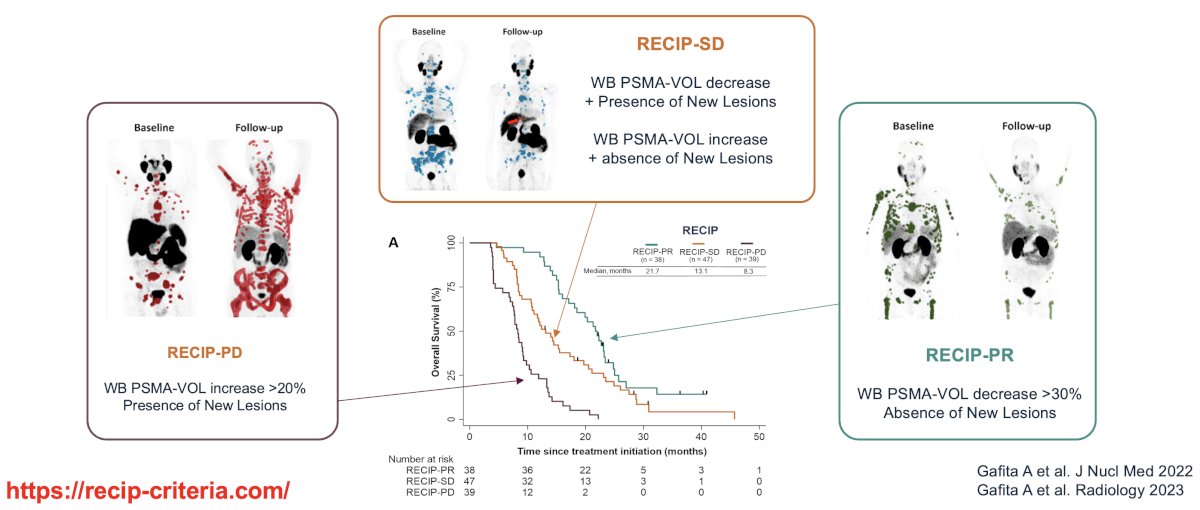
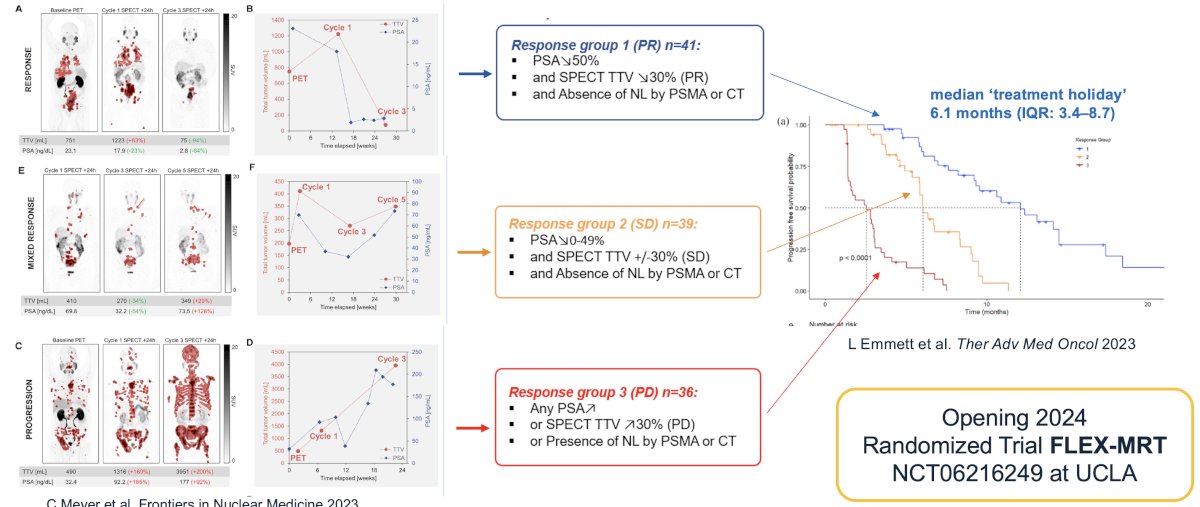
There have been numerous frameworks proposed to assess progression/response criteria, including the Response Evaluation Criteria in PSMA-imaging (RECIP) criteria.7 This system relies on whole-body PSMA volume changes and presence/absence of new lesions to define those with RECIP partial response, stable disease, or progressive disease.
The combination of 177Lu-PSMA SPECT imaging whole body volume and PSA kinetics has been proposed as a framework to monitor response to therapy. This combinatory approach will be used as the framework for dosing schedule in the FLEX MRT trial (NCT06216249). This randomized phase 2 trial compares a group of patients treated with LuPSMA on a flexible and extended dosing schedule including "treatment holiday" periods (investigational arm, up to 12 cycles) to a control group treated with a fixed dosing schedule of 6 treatment cycles maximum administered every 6 weeks. The flexible dosing schedule in the investigational arm will be based on SPECT/CT response assessments obtained 24 hours after the injection of LuPSMA therapy cycle. The response assessment during the treatment holiday period will be based on PET/CT every 12 weeks.
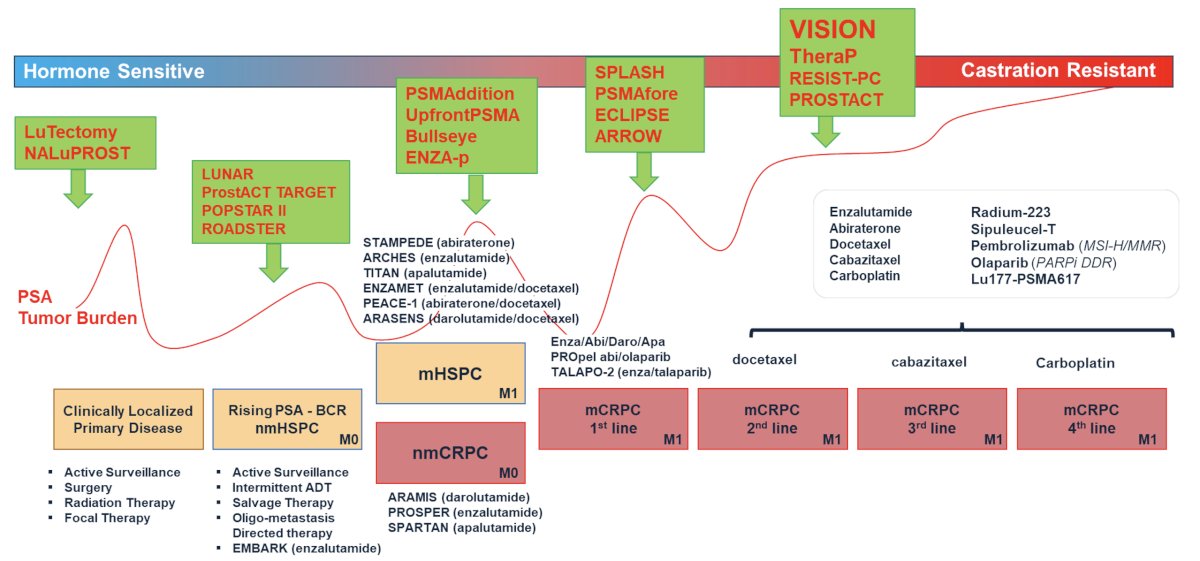
Dr. Calais noted that there is growing interest in moving these agents further up the treatment paradigm, as illustrated below. SPLASH and PSMAfore are two notable trials evaluating Lu-PSMA in the post-ARPI, pre-taxane chemotherapy setting. PSMAddition and ENZA-p are two notable trials evaluating treatment intensification with Lu-PSMA addition to doublet therapy in the mHSPC setting. Trials of neoadjuvant therapy in patients with clinically localized disease include LuTectomy and NALuPROST.
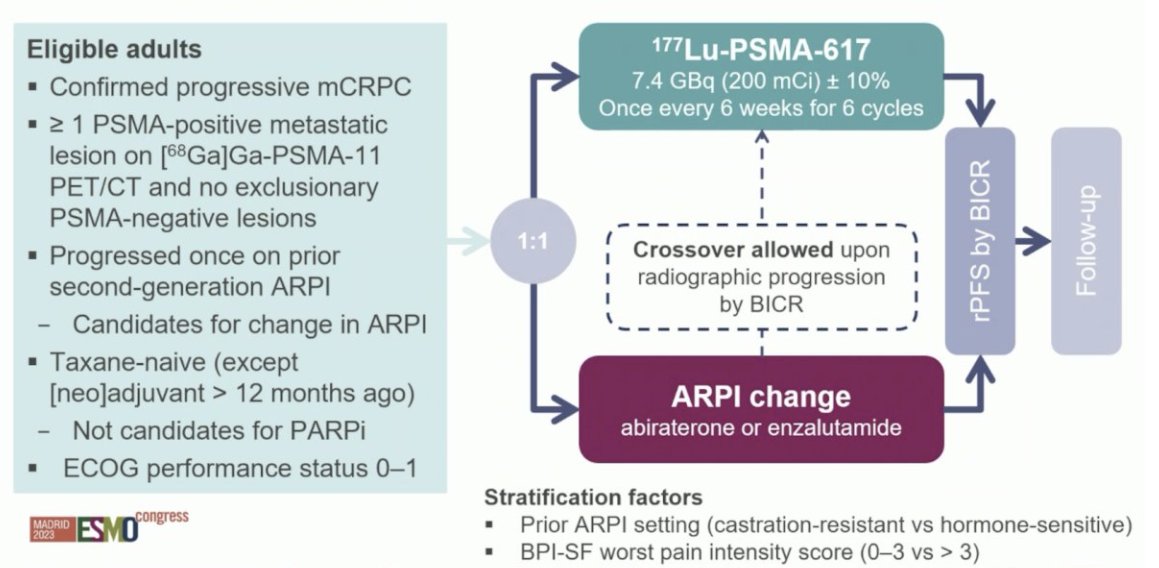
PSMAfore is a phase III trial that randomized mCRPC patients with ≥1 PMSA positive lesion and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows:
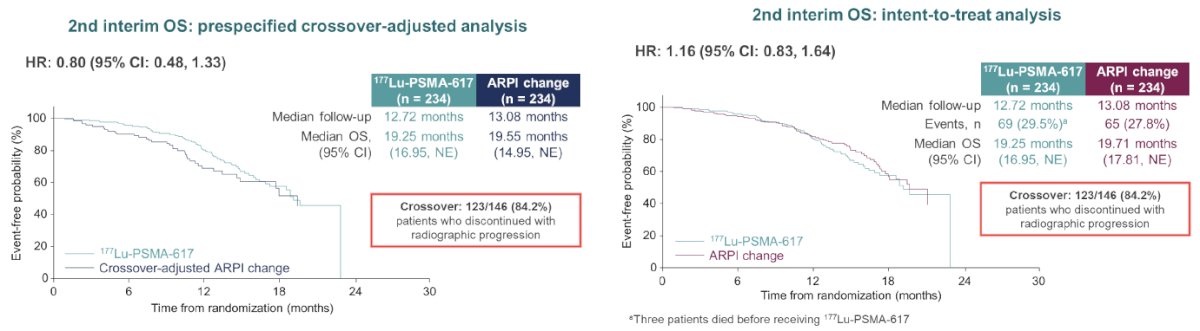
Overall, there were 468 patients randomized. At the primary analysis (median follow-up, 7.3 months), the primary endpoint of radiographic progression-free survival was met (HR: 0.41, 95% CI: 0.29 – 0.56), which was similar to the second interim analysis (HR: 0.43, 95% CI: 0.33 – 0.54).

Of note, 84.2% of patients in the ARPI switch control arm who experienced radiographic progression crossed over to 177Lu-PSMA-617. In the pre-specified, crossover-adjusted overall survival data analysis, there was a non-significant overall survival benefit in favor of 177Lu-PSMA-617 (HR: 0.80, 95% CI: 0.48 – 1.33). In the unadjusted, intention to treat analysis for overall survival, the HR was 1.16 (95% CI: 0.83 – 1.64).
Given the interim nature of these data, more mature overall survival data are required for FDA filing.
PSMAddition is a phase 3 trial of 177Lu-PSMA-617 + standard of care versus standard of care alone in patients with mHSPC (n=1,226). The study design is illustrated below. Recruitment for this trial was completed in 2023, with an estimated study completion date of February 2026.

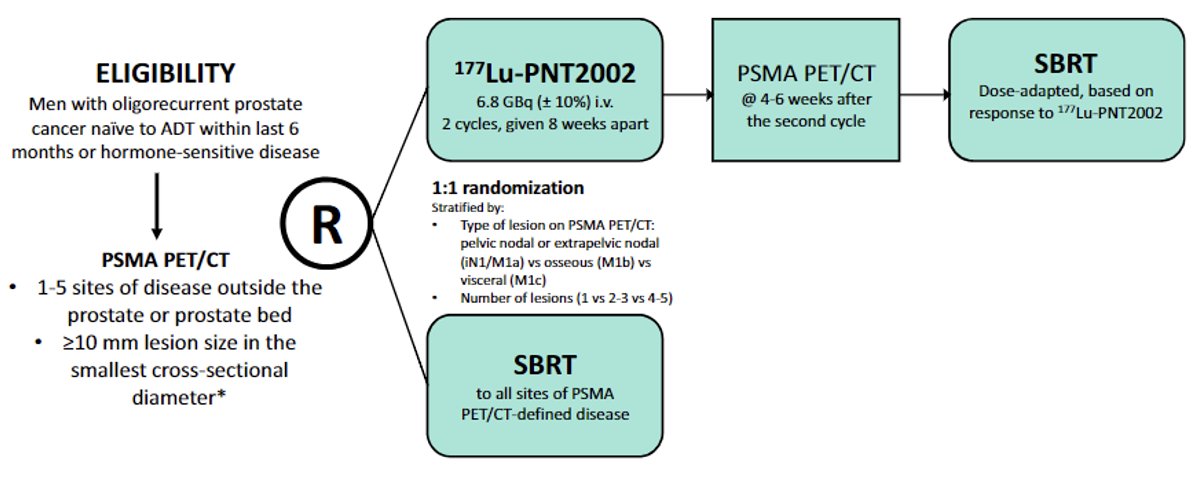
LUNAR is a randomized phase 2 trial of 177Lu-PNT2002 in patients with oligorecurrent prostate cancer planned for ablative radiotherapy. In this trial, men with oligorecurrent cancer naïve to ADT (or not received within 6 months) and 1–5 sites of disease outside the prostate or prostate bed (≥10 mm in the smallest cross-sectional diameter) are randomized to SBRT to all sites of PSMA-PET/CT-defined disease versus 177Lu-PNT2002 followed by dose-adapted SBRT based on response to 177Lu-PNT2002.

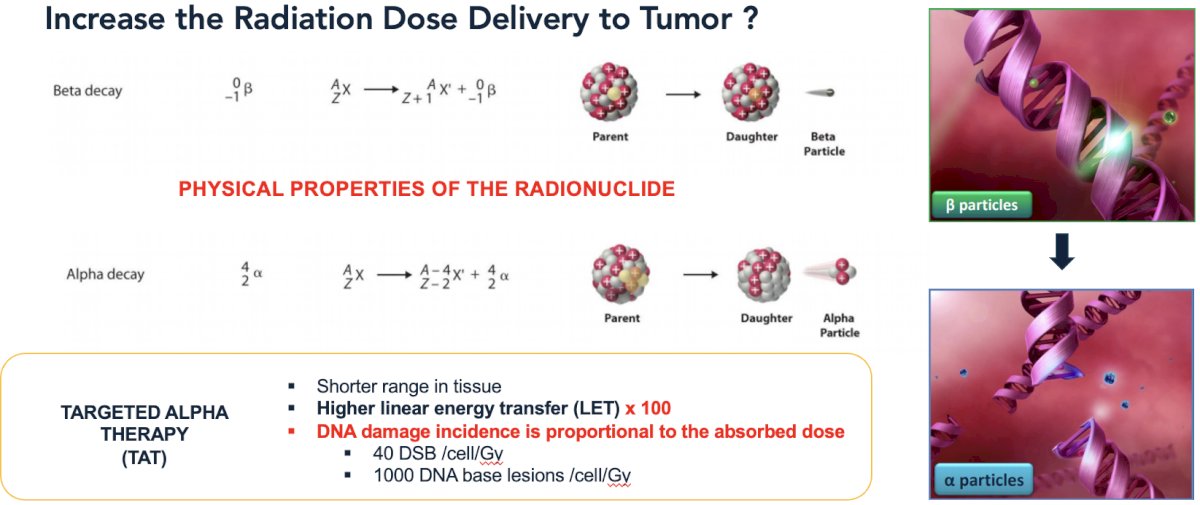
In contrast to beta emitting radionuclides, such as 177Lu-PSMA, alpha emitters, such 225Ac-PSMA have shorter tissue ranges with 100-fold higher linear energy transfer. As DNA damage incidence is proportional to the absorbed dose, this could theoretically lead to increased radiation dose delivery to the tumors.
225Ac has been evaluated over the past few years across numerous prostate cancer settings, including mHSPC, pre-chemotherapy/ARPI mCRPC settings, and in mCRPC patients with diffuse, high-volume disease.

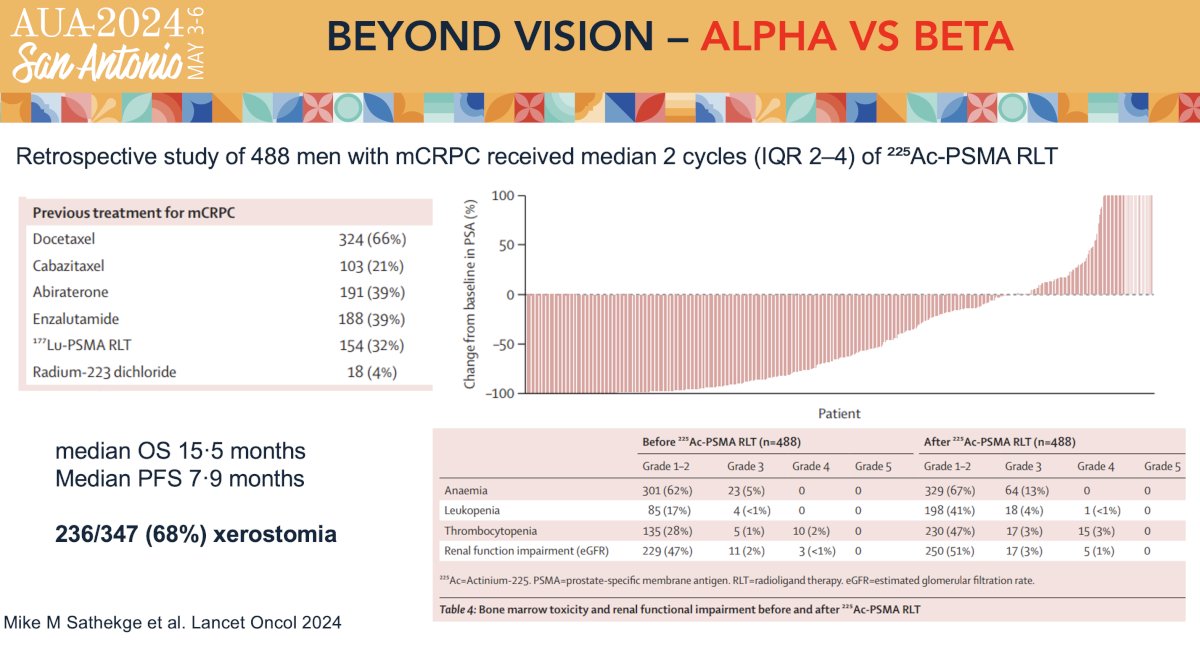
In 2024, Sathekge et al. published the results of WARMTH Act, a multicenter retrospective study of the safety and anti-tumour activity of 225Ac-PSMA radioligand therapy in mCRPC patients from seven centers in Australia, India, Germany, and South Africa. All patients in this cohort were treated with ≥1 cycle of 8 MBq 225Ac-PSMA administered intravenously. Between January 2016 and May 2023, 488 men with mCRPC received median of two cycles of 225Ac-PSMA. The median baseline PSA was 169.5 ng/ml. Previous lines of treatment included docetaxel (66%), cabazitaxel (21%), abiraterone (39%), enzalutamide (39%), 177Lu-PSMA (32%), and radium-223 (4%). The median follow-up duration was 9 months. The median overall survival was 15.5 months and median progression-free survival was 7.9 months. The most common adverse event was xerostomia, occurring in 68% of patients after the 1st cycle of 225Ac-PSMA. All patients who received more than seven cycles of 225Ac-PSMA RLT reported xerostomia. Notable grade ≥3 events included anemia (13%), leukopenia (4%), thrombocytopenia (7%), and renal toxicity (5%). There were no grade 5 events.8
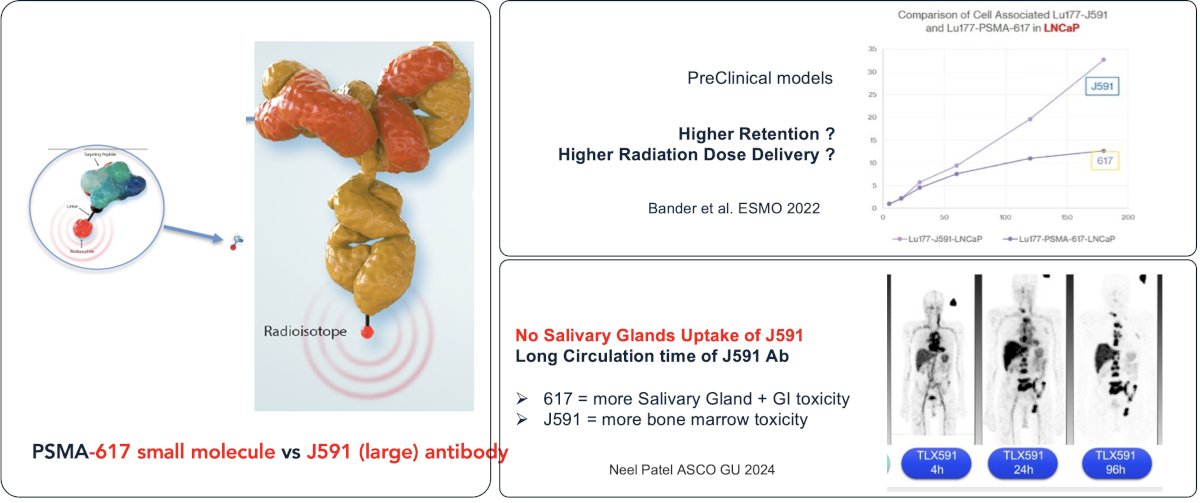
J591 is a de-immunized monoclonal antibody against the extracellular domain of PSMA and allows for accurate targeting and minor efficacy without an associated payload. The J591 monoclonal antibody may be linked to 177Lu, which is a predominantly β-emitting radionuclide with some γ-emission for imaging. J591 is significantly larger compared to PSMA-617 and is theorized that this may lead to higher retention with improved radiation dose delivery. This agent is associated with significantly lower salivary gland and gastrointestinal toxicity, at the expense of higher bone marrow toxicity.
This agent is being evaluated in PROSTACT GLOBAL, a phase 3 trial of standard of care therapy +/- 177Lu-J591in ARPI-pre-treated mCRPC patients with PSMA-positive disease.
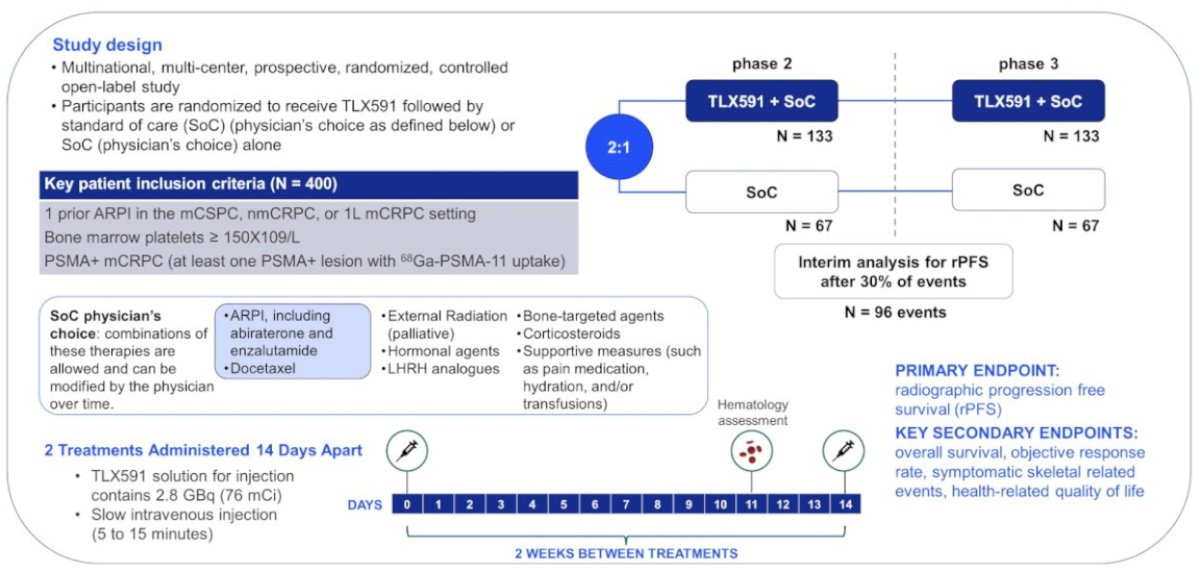
The next ‘frontier’ of radioligand therapy involves combination approaches, that aim to overcome treatment resistance mechanisms inherent to individual modalities. These include combining radionuclides with immune checkpoint inhibitors, radiosensitizers such as PARP inhibitors and chemotherapy, PSMA upregulators such as anti-androgens, and even other radionuclides (combining alpha and beta emitters)
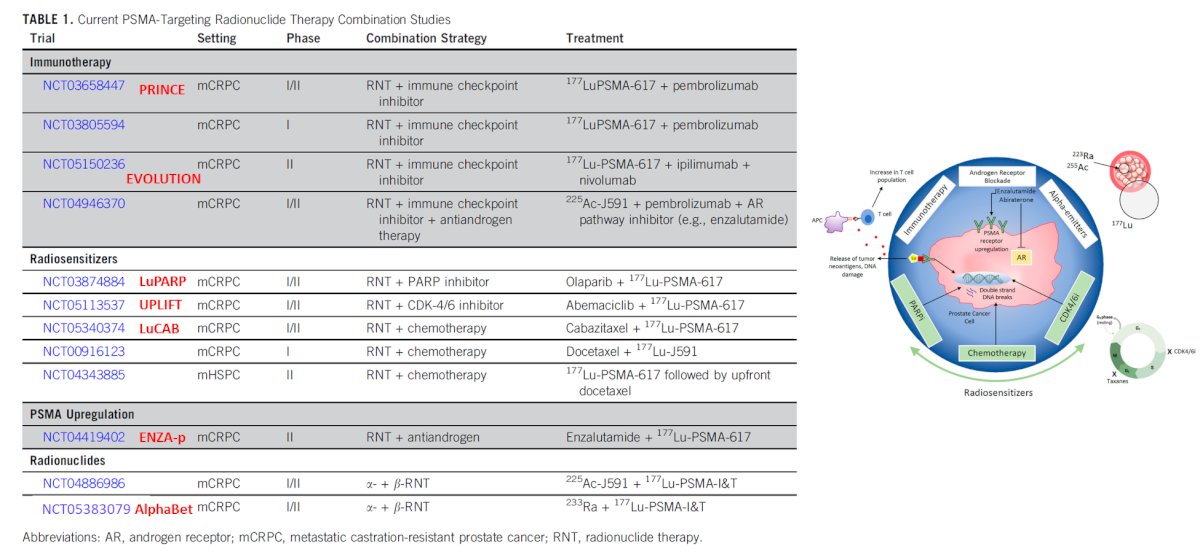
One such combination approach is 177Lu-PSMA plus enzalutamide. ENZA-p (ANZUP 1901) is an open label, randomized phase 2 trial across 15 centers in Australia of 162 mCRPC patients who had not previously received a taxane or an ARPI in the mCRPC setting, had 68Ga-PSMA-PET/CT-positive disease, and ≥2 risk factors for early progression on enzalutamide. These patients underwent 1:1 randomization to enzalutamide +/- 177Lu-PSMA-617.
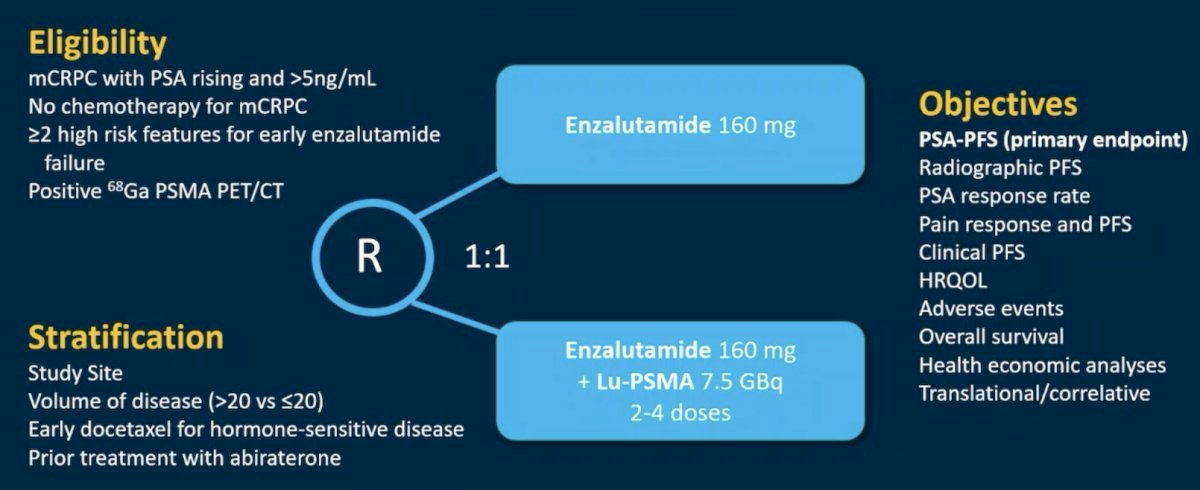
This trial met its primary endpoint, with the addition of 177Lu-PSMA-617 to enzalutamide improving PSA progression-free survival from 7.8 to 13 months (HR: 0.43, 95% CI: 0.29 – 0.63, p<0.001).9
In 2023, Aggarwal et al. published the results of a phase 1 trial of single dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in mCRPC patients. From an efficacy standpoint, 44% of patients experienced a PSA50 response and 16% a PSA90 response. 80% of patients had a response, per RECIST criteria (stable disease, partial/complete response). 26% of patients experienced a durable objective response lasting >6 months after the single dose of ¹⁷⁷Lu-PSMA. Notably, anti-tumor activity was also observed in lesions with low or negative uptake.10
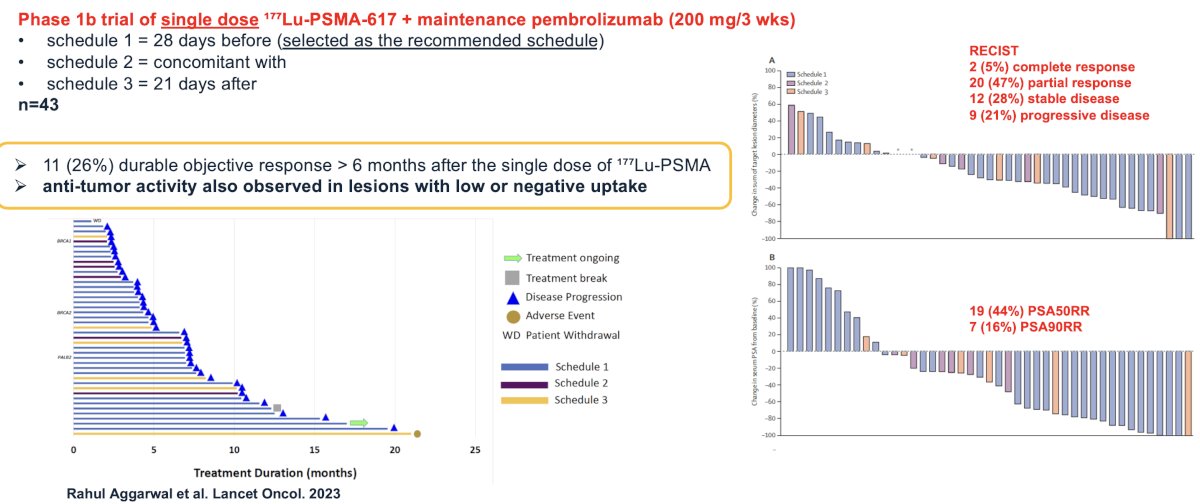
Dr. Calais concluded by highlighting the PRINCE phase 1 trial of 177Lu-PSMA-617 plus maintenance pembrolizumab in mCRPC patients. It is hypothesized that by potentially inducing immunogenic cell death, 177Lu-PSMA-617 may act synergistically with pembrolizumab, an anti-programmed death 1 inhibitor, to enhance the depth and durability of response.
mCRPC patients with high PSMA expression (SUVmax ≥ 20 in an index lesion, SUVmax > 10 for all lesions ≥ 10mm), and no FDG positive/PSMA negative lesions on paired baseline PET/CT screening, received up to 6 cycles of 177Lu-PSMA-617 (starting at 8.5 GBq, reducing by 0.5 GBq with each cycle) every 6 weeks in conjunction with 200 mg of pembrolizumab every 3 weeks for up to 2 years. The study schema is as follows:

There were 37 patients (73% had received prior docetaxel, and 100% had received a prior ARPI) that received a median of 5 cycles (range 2 to 6) of 177Lu-PSMA-617 and 12 doses (range 6 to 19) of pembrolizumab. The median follow up was 16 months, over which time the 50% PSA response rate was 76%, and 7/10 (70%) patients with RECIST-measurable disease had a partial response. The median rPFS was 11.2 months. The median PSA-progression-free survival was 8.2 months, and the median overall survival was 17.8 months.
Common (≥10%) treatment-related adverse events were mainly grade 1-2, including xerostomia (78%), fatigue (43%), pruritus (27%), nausea (27%), and rash (24%). Hematologic treatment-related adverse events included grade 2-3 anemia (8%), grade 1-2 thrombocytopenia (16%), and grade 1 neutropenia (3%). Grade 3 immune-related adverse events occurred in 10 (27%) patients with no dominant manifestation. There were 5 (14%) patients who discontinued pembrolizumab due to toxicity.

Presented by: Jeremie Calais, MD, MSc, Associate Professor of Nuclear Medicine and Theranostics, Department of Molecular and Medical Pharmacology, University of California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Benesova M, Schafer M, Bauder-Wust U, et al. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med. 2015;56(6): 914-20.
- Kratochwil C, Giesel FL, Eder M, et al. [¹⁷⁷Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(6): 987-8.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Mader N, Ngoc CN, Kirkgoze B, et al. Extended therapy with [177Lu]Lu-PSMA-617 in responding patients with high-volume metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50(6): 1811-21.
- Seifert R, Telli T, Lapa C, et al. Safety and Efficacy of Extended Therapy with [177Lu]Lu-PSMA: A German Multicenter Study. J Nucl Med. 2024.
- Gafita A, Rauscher I, Weber M, et al. Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study. J Nucl Med. 2022;63(11): 1651-8.
- Sathekge MM, Lawal IO, C Bal, et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 2024;25(2): 175-83.
- Emmett L, Subramaniam S, Crumbaker M, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024:S1470-2045(24)00135-9
- Aggarwal R, Starzinski S, de Kouchkovsky I, et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023;24(11): 1266-76.


