(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to a prostate cancer staging podium session. Dr. Michael Leapman presented the results of a retrospective cohort analysis of prostate-specific membrane antigen (PSMA) imaging findings and subsequent clinical management among patients with biochemically recurrent prostate cancer.
Biochemical recurrence following primary therapy (radical prostatectomy or external beam radiotherapy) occurs in 10–80% of prostate cancer patients. Conventional imaging techniques (CT scan, bone scintigraphy) demonstrate poor performance characteristics for staging these patients in this setting, with reported sensitivity, specificity, and accuracy of 38%, 91%, and 65%, respectively.1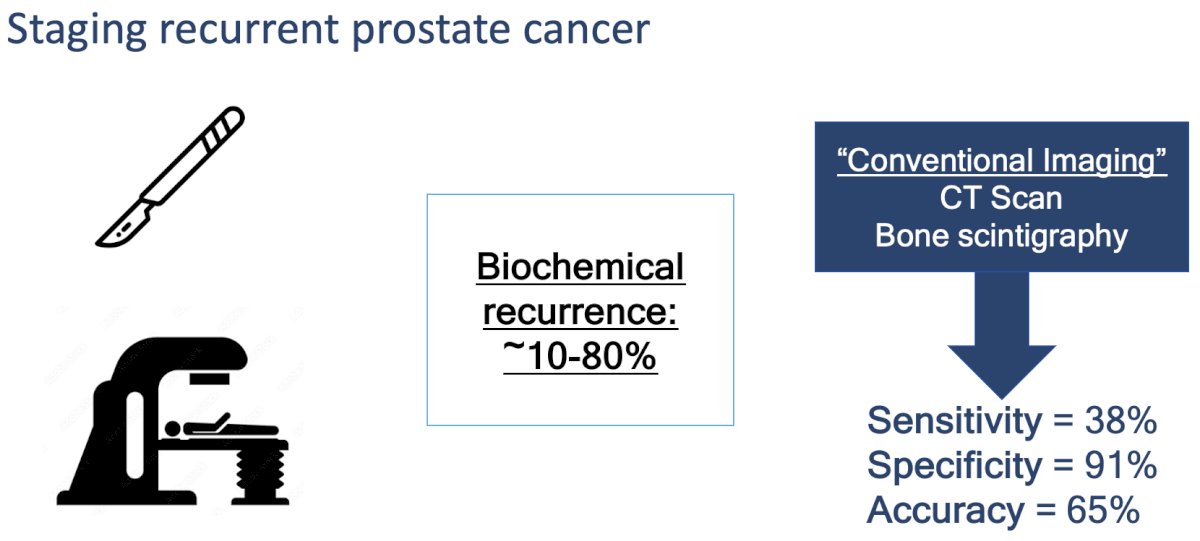
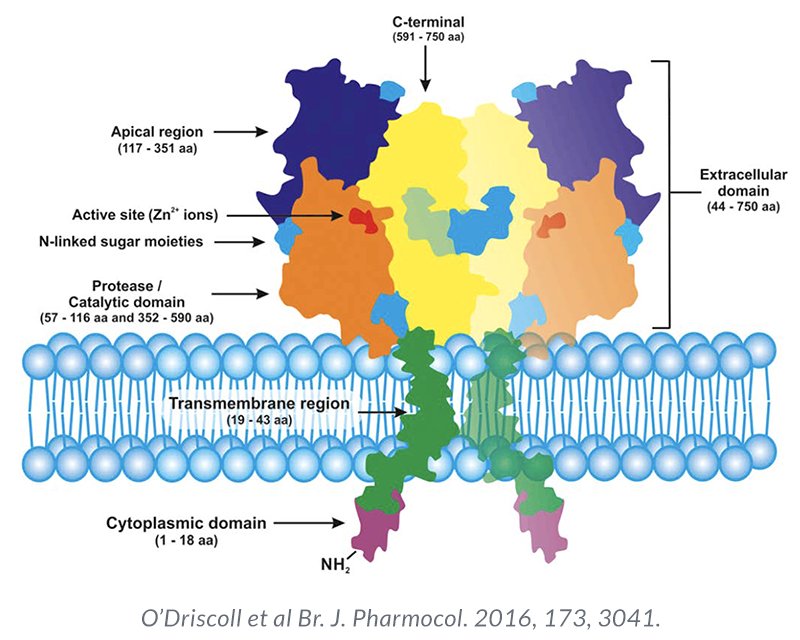
PSMA is a transmembrane protein expressed by the epithelial cells lining the proximal renal tubules, salivary glands, small bowel, as well as the prostate. Expression of this transmembrane protein is upregulated in prostate cancer cells by up to 1000-fold. The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer, thus making it highly prevalent in all forms of prostate cancer, including some castrate-resistant forms. Importantly, the relatively poor expression of PSMA in other organs allows for enhanced targeted imaging in prostate cancer patients.2 Compared to conventional imaging, PSMA-PET/CT demonstrates superior performance characteristics in the biochemically recurrent setting:3
- Sensitivity: 85%
- Specificity: 98%
- Accuracy: 92%
- Biochemical recurrence localization: 85–87%
Illustrated below is the timeline of FDA approvals of molecular imaging agents for prostate cancer:
The objective of this study was to evaluate clinical management in the six-month period following first PSMA-PET scan in relation to imaging findings in the era following approval of PSMA-PET in the United States (US).
The study investigators conducted a retrospective cohort study of patients undergoing PSMA-PET imaging for the evaluation of biochemical recurrence from two large US academic referral centers.
They classified treatment categories as no treatment, local therapy, systemic therapy, and metastasis-directed therapy (MDT). In addition, they evaluated PSMA-PET findings and subsequent therapy by PSA strata at imaging (0-1.99, 2.0-4.99 and ≥5.0 ng/mL).
This study included 583 patients undergoing PSMA-PET imaging from March 2019 through February 2023, including 393 (67%) with prior prostatectomy, 155 (27%) radiation therapy, and 35 (6%) with other treatments. The median PSA at testing was 1.8 ng/ml.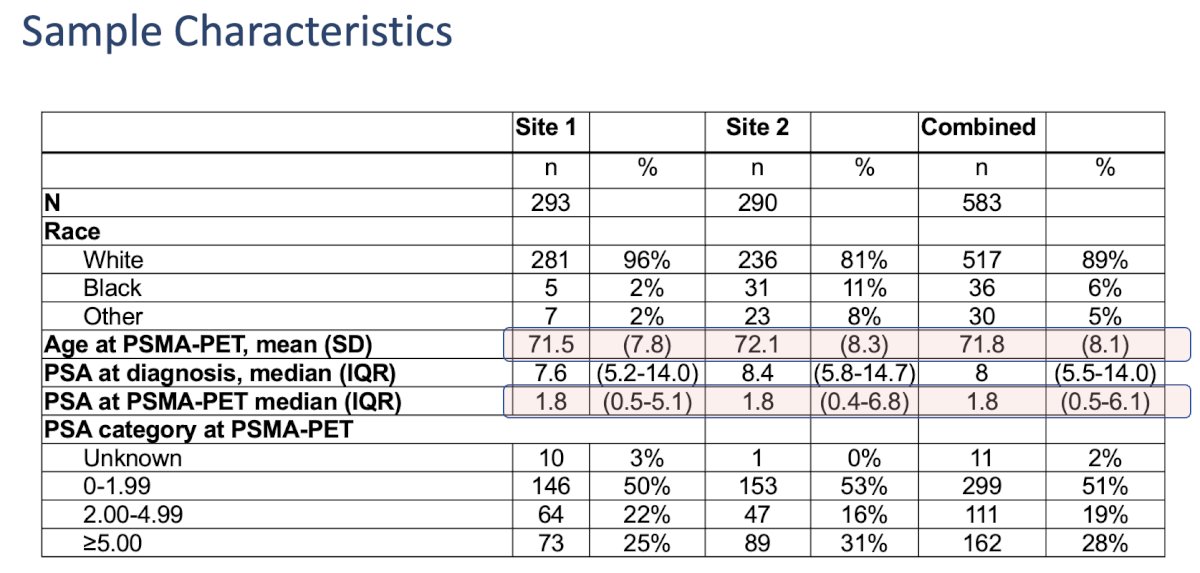
Overall, 76% of patients had PSMA-positive findings:
- Localized recurrence: 15%
- Regional pelvic nodal disease: 10%
- Distant metastasis: 50%
- Distant metastasis was identified in 35% of those with PSA 0-1.99, 60% with PSA 2.0-4.99, and 72% of those with PSA >5 ng/ml.
Subsequent management within six months of imaging differed by PSMA finding. Among 142 patients with negative imaging, 63 (44%) received any treatment. Conversely, 93% of patients with detected metastases were treated, including with systemic therapy in 189 (64%), metastasis-directed therapy (MDT) in 76 (26%), and local therapy alone in 7 (2%).
Distributions of treatment differed by PSA level within the strata of PSMA findings. For example, among 294 patients with metastases identified, MDT was used more frequently in those with PSA <2.0 ng/mL (37%) as compared with 2.0-4.99 ng/mL (30%) or PSA ≥5.0 ng/mL (14%), p<0.001.
Dr. Leapman concluded that:
- In this retrospective study of patients undergoing PSMA-PET imaging for evaluation of biochemical recurrence in the early period following approval of PSMA imaging tracers, most patients were found to have identifiable areas of disease and initiated therapy within six months.
- Limitations include absence of a comparator group and non-controlled indications for testing
Presented by: Michael Leapman, MD, MHS, Associate Professor of Urology, Department of Urology, Yale School of Medicine, New Haven, CT
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024
References:
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-center, single-arm phase 2 study. Lancet Oncol 2018 Jun;19(6):825-833.
- Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga] gallium-labeled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumor lesions. Eur J Nucl Med Mol Imaging 2013;40:486-95.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.


