(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to the International Prostate Forum. Dr. Stacy Loeb discussed the role of the urologist in genetic testing for prostate cancer.
In 2016, Pritchard et al. demonstrated in a cohort of 692 men that 12% of men with metastatic prostate cancer have germline mutations in DNA repair genes. Notably, 5% of men with localized prostate cancer also harbor such germline mutations.1
A potential benefit of genetic testing in prostate cancer is that it may influence screening and treatment protocols. It may shed information on the personal and familial risk of prostate cancer, as well as other malignancies such as breast cancer.
The urologist plays an important role in genetic testing by identifying who needs genetic evaluation. What are some criteria that are associated with an increased risk of underlying germline mutations?
- Tumor characteristics
- High-risk localized, very high-risk localized, regional (node positive) or metastatic
- Consider for intermediate-risk with cribriform/intraductal histology
- Past medical history
- Personal history of breast cancer
- Consider for exocrine pancreatic, colorectal, gastric, melanoma, UTUC, glioblastoma, biliary tract, and small intestinal
- Family history/ancestry
- Known familial history of high-risk mutation (e.g., BRCA2, BRCA1, ATM, PALB2, CHEK2, MLH1, MSH6, PMS2, EPCAM)
- ≥1 close relative with breast cancer (triple-negative or age ≤50 in female, or male at any age), ovarian or pancreatic cancer at any age, colorectal or endometrial cancer at age ≤50, prostate cancer at age ≤60 or high-risk/metastatic prostate cancer
- ≥2 close blood relatives with breast or prostate cancer (any grade, any age)
- ≥3 first or second degree relatives with Lynch syndrome-related cancers, especially if diagnosed at age <50
- Ashkenazi Jewish ancestry
There are numerous guides available to help determine who needs genetic evaluation, including the HELIX App, developed by Dr. Loeb’s team:2


After the urologist identifies who might benefit from genetic testing, the next step is to offer genetic testing when indicated or refer to a genetic counselor. As outlined in the 2019 Philadelphia Consensus Conference, there are important elements of informed consent for germline testing, namely:
- Purpose of germline testing
- Possibility of uncovering hereditary cancer syndromes
- Panel options
- Potential types of test results
- Potential to uncover additional cancer risks
- Potential out-of-pocket cost
- Genetic information Nondiscrimination Act law and other laws that address genetic discrimination
- Cascade testing/additional familial testing
- Data-sharing/data-selling policies of genetic laboratories
- Privacy of genetic tests
Overall, it appears that prostate cancer patients are satisfied by the germline genetic counseling they receive from their clinicians. In a survey of 275 men with metastatic or localized prostate cancer meeting National Comprehensive Cancer Network® criteria for consideration of genetic testing, such men were offered pre-test genetic counseling by their urologist or medical oncologist as part of their routine clinical care and concurrently approached for enrollment in the Germline Genetics in Prostate Cancer Study. Consented patients filled out a post-counseling survey using validated instruments to assess the quality of counseling. A total of 275 patients enrolled, of whom 203 elected to undergo genetic testing. Patient satisfaction was high, with 98% of patients reporting being satisfied with the overall quality of pre-test counseling, and 74% of patients electing to undergo genetic testing.3
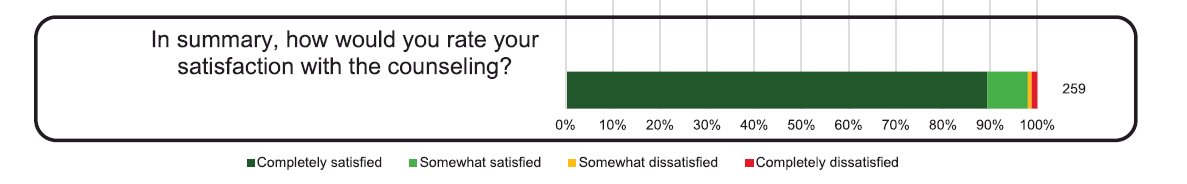
One notable barrier to germline genetic testing in these patients is the availability of genetic counselors at the institutional or geographical regional level. The National Society of Genetic Counselors (NSGC) tool helps patients and clinicians find in-person and telehealth options to overcome such shortcomings.
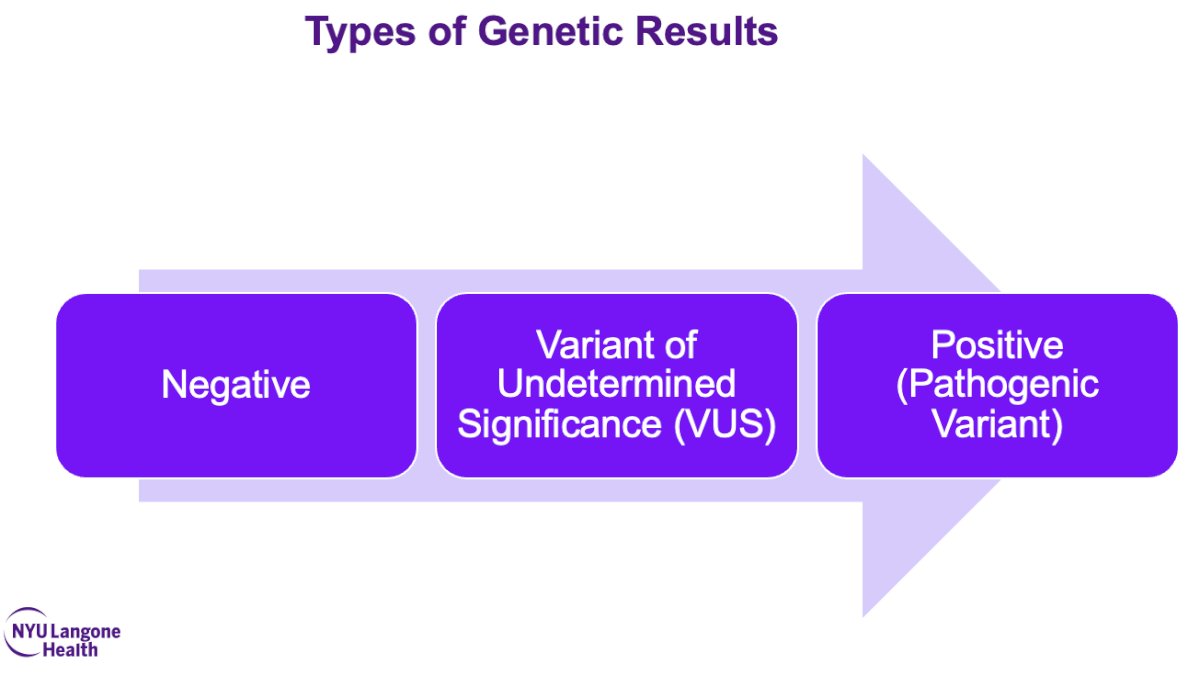
After ordering the genetic testing (or referring to the genetic counselor), the next step for the urologist is to evaluate the results, which can be ‘negative’, ‘variant of undetermined significance’, or ‘positive (pathogenic variant)’.
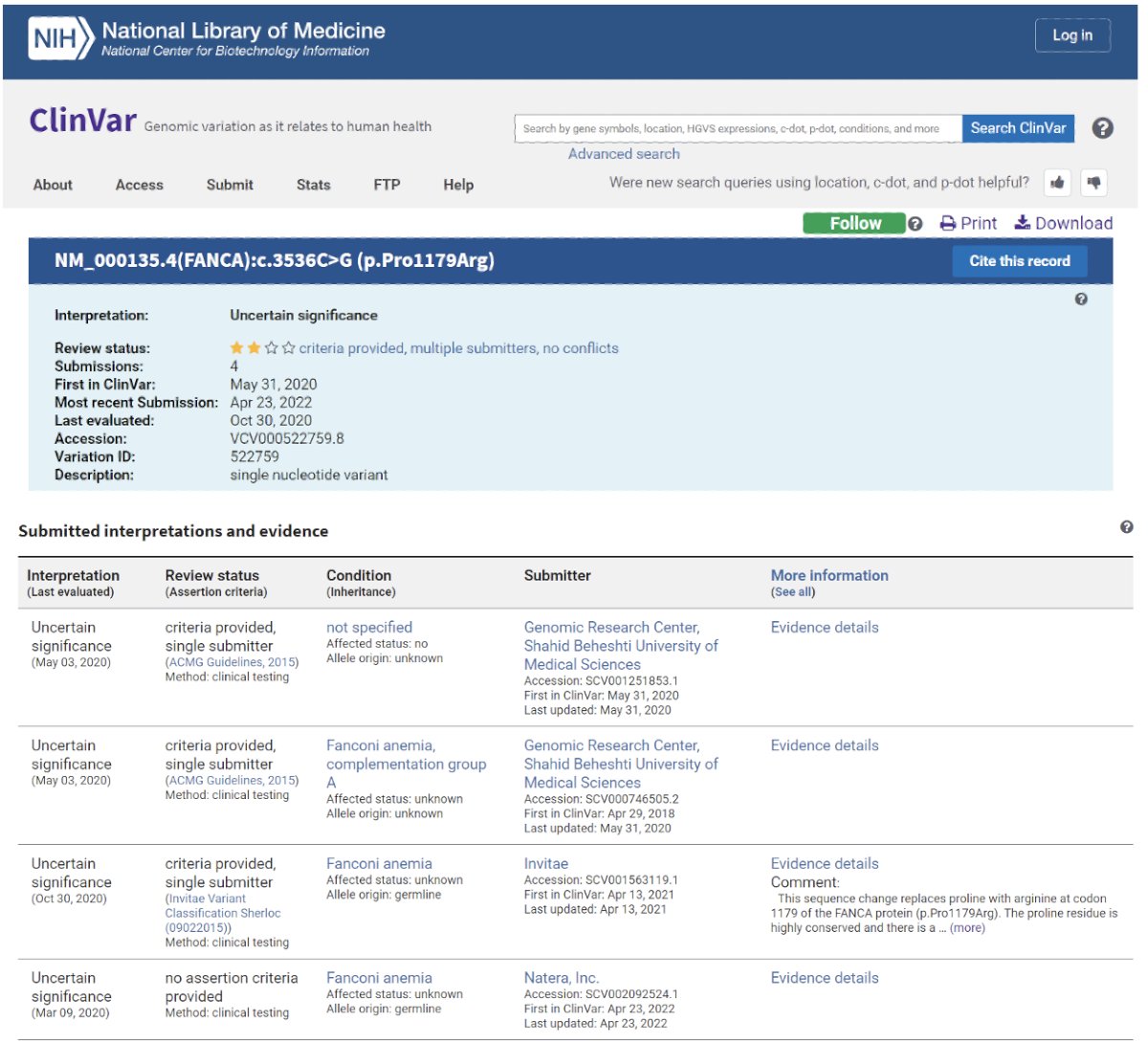
Imaged below is an example of a genetic testing report generated by ClinVar and interpreted by a clinician in practice:
The next step for the urologist is to use the results for prostate cancer screening purposes. What is the impact of positive germline testing findings on prostate cancer screening? The 2019 Philadelphia Prostate Cancer Consensus Conference recommends early prostate cancer detection starting at age 40 or 10 years before the youngest prostate cancer diagnosis in the family when BRCA2 mutations are present and to be considered when BRCA1, HOXB13, ATM, and DNA mismatch repair mutations are present.

The IMPACT study evaluated targeted prostate cancer screening using PSA in men with germline BRCA1/2 mutations. Men aged 40–69 years with a germline pathogenic BRCA1/2 mutation and male controls testing negative for a familial BRCA1/2 mutation were recruited. Participants underwent PSA screening for three years, and if PSA was > 3 ng/ml, men were offered a prostate biopsy. Cancer incidence rate per 1,000 person years was higher in BRCA2 carriers than in noncarriers (19.4 vs 12.0; p=0.03); BRCA2 carriers were diagnosed at a younger age (61 vs 64 years; p = 0.04) and were more likely to have clinically significant disease than BRCA2 non-carriers (77% vs 40%; p=0.01).4 A similar approach evaluating MSH2/MSH6 carriers versus non-carriers demonstrated a higher cancer incidence and frequency of clinically significant disease.5
What are the suggested PSA thresholds for action in BRCA1/2 germline carriers? Cheng et al. have proposed the following thresholds based on patient age:6

Dr. Loeb highlighted the ongoing NCI trial for screening with PSA and MRI in men at high genetic risk for prostate cancer and encouraged the audience to refer patients, where possible.
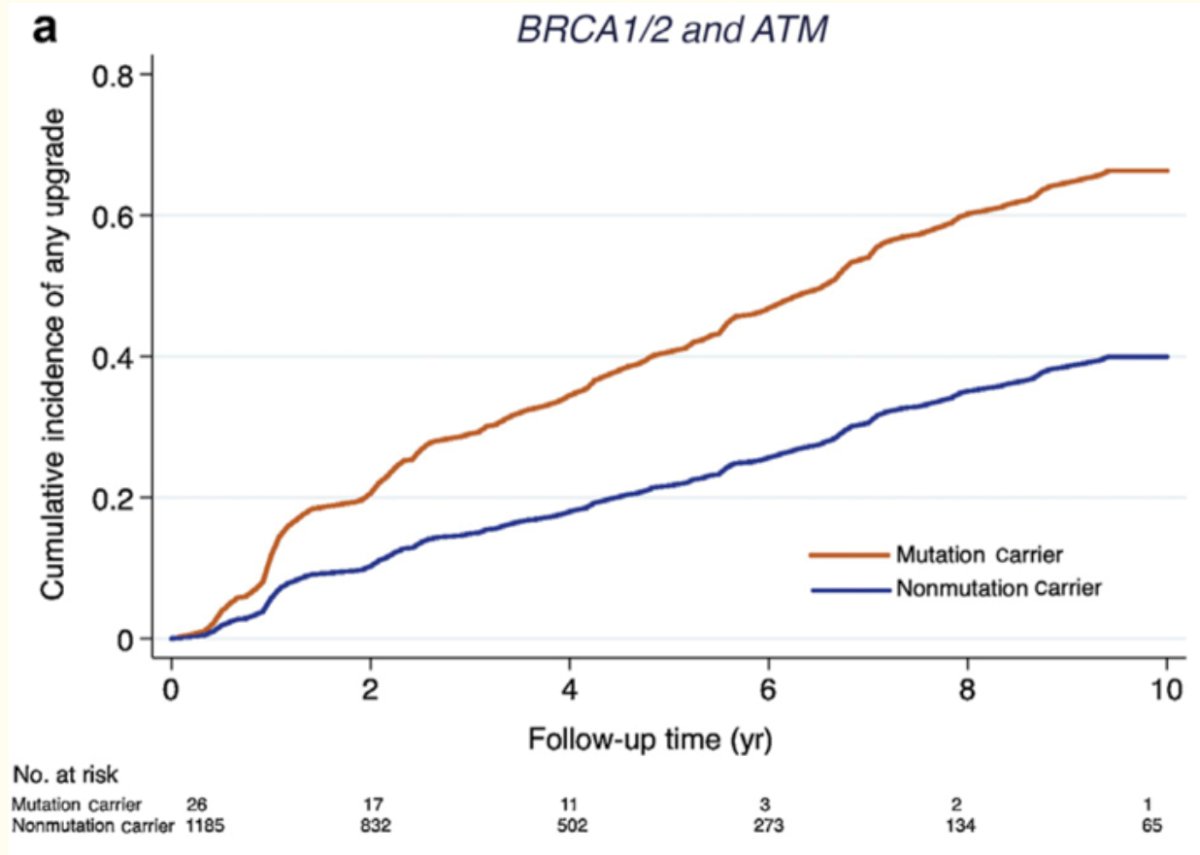
How can urologists use the results of germline genetic testing to inform prostate cancer treatment? Men with BRCA/ATM pathogenic variants are at higher risk of re-classification during active surveillance, which suggests that these patients should either undergo more intense surveillance or be considered for definitive therapy options.7
Interestingly, Brady et al. have demonstrated in a cohort of active surveillance patients that carrier rates of pathogenic germline mutations in ATM, BRCA1, and BRCA2 do not differ in patients with or without grade reclassification (1.9% vs. 1.8%).8
In patients with metastatic castration-resistant prostate cancer, numerous PARP inhibitors have emerged as a treatment option in both earlier and later line treatment settings.

What are some additional tools for urologists and patients? Media resources include the Prostate Cancer Genetics Podcast co-hosted by Dr. Loeb and supported by the Department of Defense. This podcast addresses topics such as important genes involved in prostate cancer, genetic testing, and precision medicine, with speakers including physicians, genetic counselors, patients, and families.

Virtual genetics board meetings that address cases discussions highlighting clinical management, genetic counseling, genetic testing, hereditary cancer management, and complexities are also virtually available (www.prostategenetics.com/engagement).
The TARGET (Technology-enhanced AcceleRation of Germline Evaluation for Therapy) study is a randomized non-inferiority trial of a pre-test, patient-driven genetic education webtool versus genetic counseling for prostate cancer germline testing. This educational tool was jointly developed with the Prostate Cancer Foundation and includes 9 genetic education modules with quizzes to ensure understanding.

Dr. Loeb concluded by noting the following:
- Germline mutations are found in a greater proportion of prostate cancer than previously recognized
- Germline test results have important implications for screening and treatment of prostate cancer, as well as important implications for personal and family cancer risk and clinical trial eligibility.
- Technological tools may assist with implementation of germline testing in practice.
- Refer to genetic counselor for support with counseling and/or management of results (The National Society of Genetic Counselors website has tool to find a counselor)
Presented by: Stacy Loeb, MD, Professor of Urology and Population Health at NYU Langone Health and the Manhattan Veterans Affairs, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- Giri VN, Walker A, Gross L, et al. Helix: A Digital Tool to Address Provider Needs for Prostate Cancer Genetic Testing in Clinical Practice. Clin Genitourin Cancer. 2022;20(2): e104-13.
- Abusamra SM, Solorzano MA, Luke M, et al. Satisfaction With Clinician-Led Germline Genetic Counseling in Patients With Prostate Cancer. J Urol. 2022;208(5): 1007-17.
- Page EC, Bancroft EK, Brook MN, et al. Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur Urol. 2019;76(6): 831-42.
- Bancroft EK, Page EC, Brook MN, et al. A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study. Lancet Oncol. 2021;22(11): 1618-31.
- Cheng HH, Pritchard CC, Montgomery B, Lin DW, Nelson PS. Prostate Cancer Screening in a New Era of Genetics. Clin Genitourin Cancer. 2017;15(6): 625-8.
- Carter HB, Helfand B, Mamawala M, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol. 2019;75(5): 743-9.
- Brady L, Newcomb LF, Zhu K, et al. Germline mutations in penetrant cancer predisposition genes are rare in men with prostate cancer selecting active surveillance. Cancer Med. 2022;11(22): 4332-40.


