(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to an advanced prostate cancer moderated poster session. Dr. Benjamin Lowentritt presented a study evaluating darolutamide real-world doublet and triplet utilization in metastatic hormone sensitive prostate cancer (mHSPC), within the context of a US community urology setting.
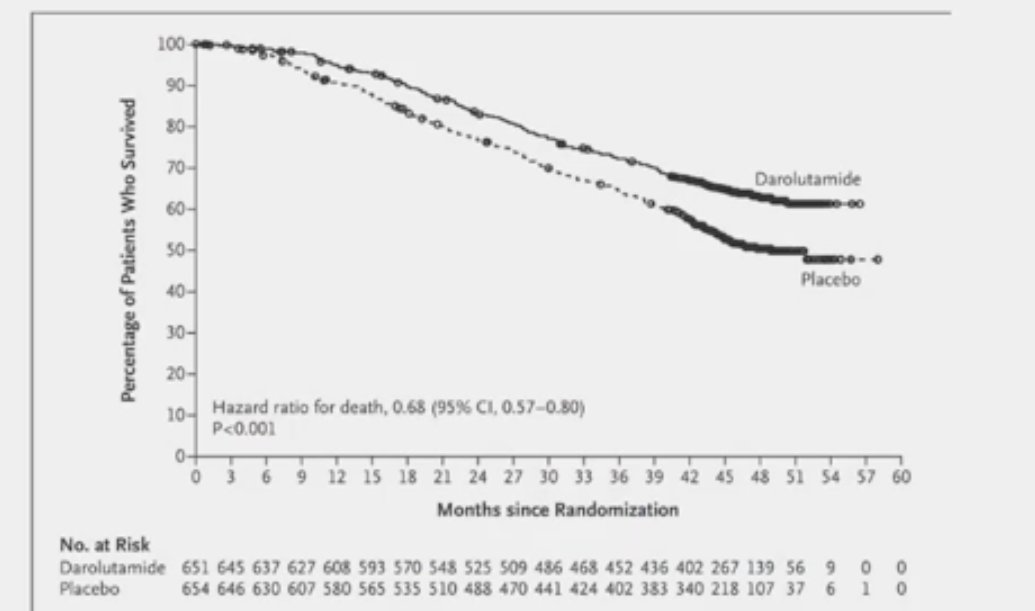
Triplet therapy with darolutamide plus ADT and docetaxel is currently FDA approved and has received an NCCN Guidelines category 1 recommendation for the treatment of mHSPC patients. This follows the results of the ARASENS trial that demonstrated a significant 32.5% decreased rate of death among patients treated with darolutamide + ADT + docetaxel, compared to placebo + ADT + docetaxel (HR: 0.675, 95% CI 0.568–0.801; P< 0.0001).1
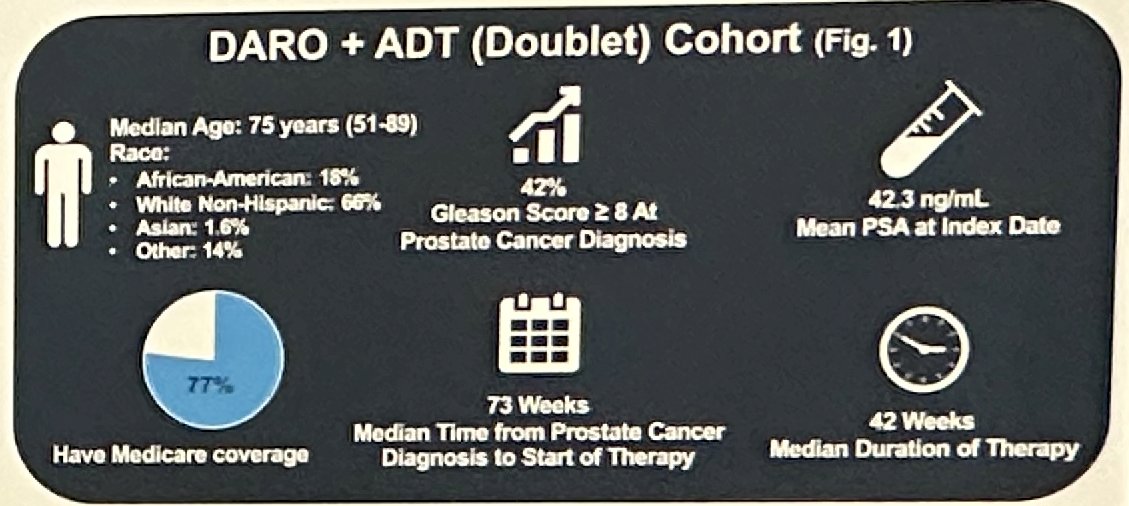
To date, there are limited data on the real-world utilization and treatment patterns of darolutamide plus ADT (doublet therapy) for mHSPC patients. The primary objective of this analysis was to describe the clinical profile of those receiving darolutamide doublet therapy.
This was a retrospective analysis using the largest urology integrated database in the United States, PPS Analytics (Specialty Networks) Patient Population Health Management Platform composed of 40 community urology practices. Data acquisition included structured data in electronic medical records and chart abstraction for data in clinical notes. The study included 319 men with de novo and recurrent mHSPC treated with darolutamide doublet or triplet therapy between January 1, 209 and March 31, 2023.
Of the 319 eligible mHSPC patients, 192 (60%) received darolutamide doublet therapy and 127 (40%) received triplet therapy. In the doublet therapy cohort, the median age was 75 years, 18% were African American, and 66% were White Non-Hispanic. The majority of patients (77%) had Medicare coverage. Nearly half of doublet therapy patients (42%) had a high Gleason score (≥8) and the mean PSA at start of therapy was 42.3 ng/ml. The majority of patients (83%) initiated ADT followed by darolutamide and the median time from prostate cancer diagnosis to start of therapy was 73 weeks.
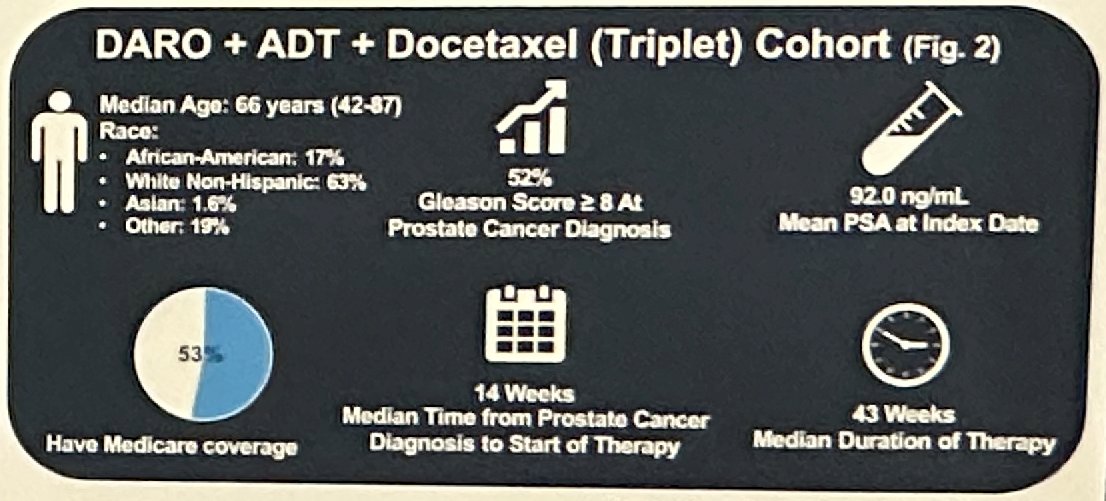
In the triplet therapy group, the median age was 66 years. 63% and 17% were White non-Hispanic and African American, respectively. 53% of patients had Medicare coverage. The Gleason Score at diagnosis was ≥8 in 52% of patients. The mean PSA at diagnosis was 92 ng/ml. The median time from prostate cancer diagnosis to start of therapy was 14 weeks.
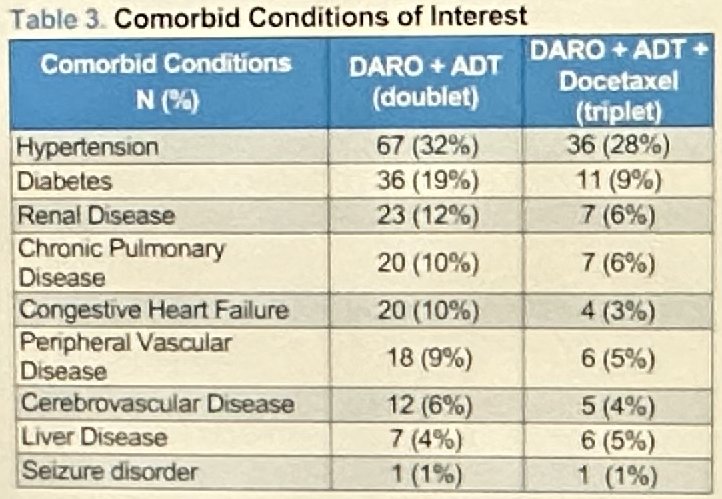
Overall, patients in the triplet therapy group generally had a more favorable comorbidity profile:
Dr. Lowentritt concluded that results of this community-representative urology database demonstrate that darolutamide doublet and triplet therapy are being adopted in clinical practice. Furthermore, most patients also possessed unfavorable disease characteristics including high Gleason Grades and PSA values. These data warrant additional study of treatment patterns in mHSPC as patient characteristics continue to evolve.
Presented by: Benjamin H. Lowentritt, MD, FACS, Urologist, Chesapeake Urology Associates PA, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:


