(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between was host to an advanced prostate cancer moderated poster session. Dr. Keita Nakane presented an analysis of trends in the use of second-generation androgen receptor axis inhibitors for metastatic hormone-sensitive prostate cancer (mHSPC) and clinical factors predicting biologic recurrence.
Although numerous androgen receptor pathway inhibitors (ARPIs) are currently available for the treatment of mHSPC patients, to date, no randomized controlled trials directly comparing the efficacy and safety of ARPIs have been conducted. It remains unclear how treatment patterns have changed in real-world practice since 2020 when ARPIs became available for use in patients with mHSPC in Japan. Dr. Nakane and colleagues conducted a multicenter retrospective study to evaluate patient characteristics and oncologic outcomes as well as to examine clinical factors that predict biochemical recurrence-free survival.
The study investigators retrospectively analyzed 581 newly diagnosed patients with previously untreated, histologically confirmed mHSPC who were started on hormone therapy between February 2018 and December 2021. All mHSPC patients underwent computed tomography (CT) of the chest, abdomen, and pelvis, as well as magnetic resonance imaging (MRI) of the pelvis, and bone scintigraphy to evaluate pre-treatment baseline characteristics.
The patients were categorized based on the specific ARPI received: abiraterone, apalutamide, and enzalutamide. Biochemical or disease progression was defined per the Prostate Cancer Clinical Trials Working group 3 criteria. High-risk disease was defined using LATITUDE criteria (≥2 of the following 3 criteria):
- ≥3 bone metastases by bone scintigraphy
- Gleason score ≥8
- Presence of visceral metastases
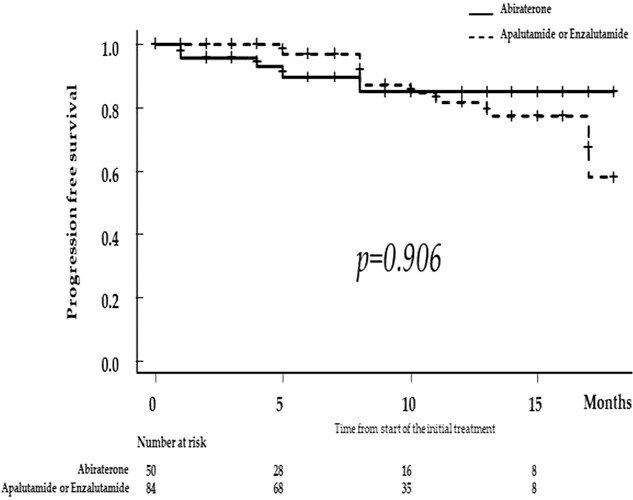
There was an increase in the utilization of ARPIs following May 2020. There were no significant differences in biochemical recurrence-free survivals between patients receiving apalutamide versus enzalutamide (p=0.49) or apalutamide (p=0.91).

Multivariable analysis revealed that a neutrophil-to-lymphocyte ratio ≥2.76 and PSA ≥0.55 ng/mL were independent predictors of biochemical recurrence. As demonstrated in the Kaplan Meier curve below, patients with variables present had significantly worse biochemical recurrence-free survival compared to those with 0–1 predictors present.

Dr. Nakane concluded that this study demonstrates no significant differences in biochemical recurrence-free survival rates by type of ARPI used for the treatment of mHSPC patients. A neutrophil-to-lymphocyte ratio ≥2.76 and PSA ≥0.55 ng/ml are both significant predictors of biochemical recurrence-free survival in this setting. These results need further validation in large prospective studies.
Presented by: Keita Nakane, PhD, Assistant Professor, Department of Biochemistry, Albert Einstein College of Medicine, Bronx, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


