Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor with low blood-brain barrier penetration and limited potential for drug-drug interactions. In patients with nmCRPC, darolutamide significantly improved median metastasis-free survival by ~2 years and reduced the risk of death by 31% compared to placebo, with a favorable tolerability profile in the phase 3 ARAMIS trial.1,2
DAROL (NCT04122976) is assessing the real-world safety and effectiveness of darolutamide in patients with nmCRPC. In this report, Dr. Gotto presented the pre-specified 3rd interim analysis of this study.
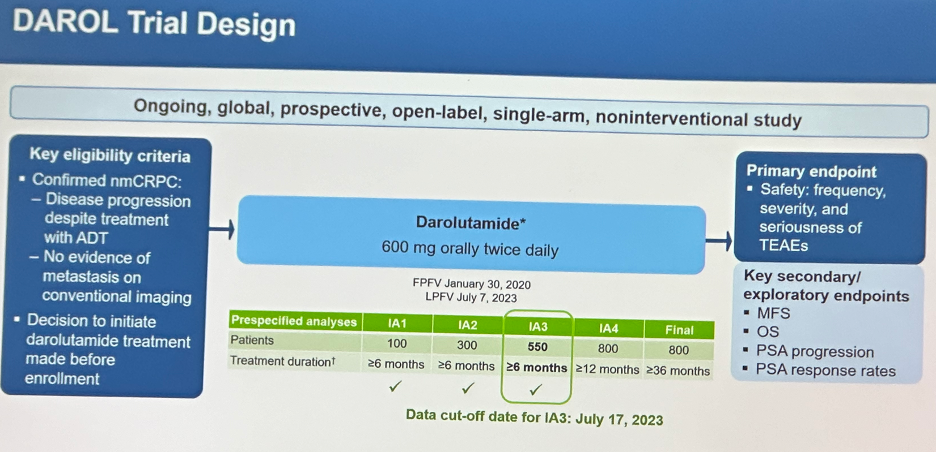
DAROL is an ongoing, global, prospective, open-label, single-arm, non-interventional study that included patients with confirmed nmCRPC and for whom the decision was made to initiate darolutamide treatment prior to enrollment. Patients received darolutamide 600 mg orally twice daily. The primary endpoint of this study was safety events (frequency, severity, and seriousness of treatment-emergent adverse events). Key secondary endpoints included:
- Metastasis-free survival
- Overall survival
- PSA progression
- PSA response rates
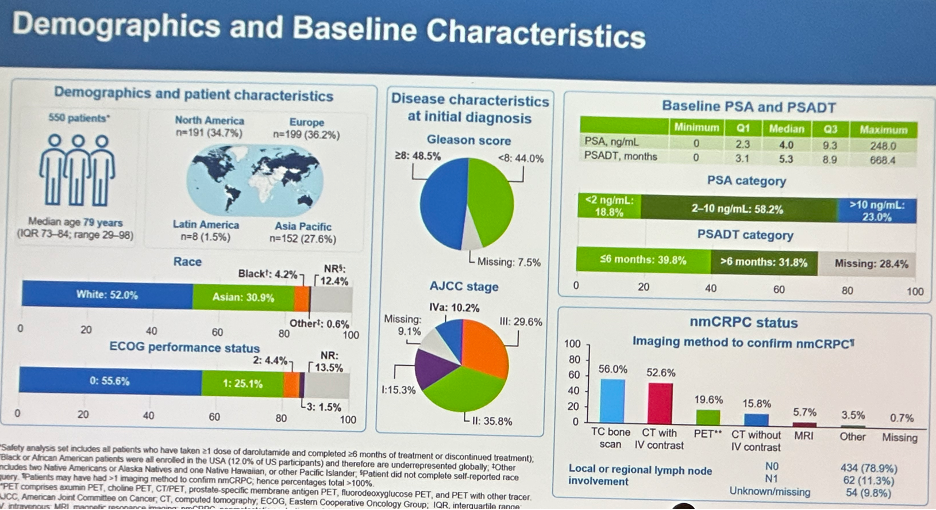
The demographics and baseline characteristics of the study cohort (n=550) are summarized below. The median patient age was 79 years. Patients were recruited from North America (34.7%), Europe (36.2%), Asia Pacific (27.6%), and Latin America (1.5%). The majority of patients were either White (52%) or Asian (31%). 80% had an ECO performance status of 0–1. With regards to disease characteristics at initial diagnosis, 48.5% of patients had Gleason Score ≥8 disease. The median baseline PSA was 4 ng/ml and the median PSA doubling time was 5.3 months. Although nmCRPC Is defined using conventional imaging techniques, 20% of patients had their non-metastatic status confirmed using a PET scan.
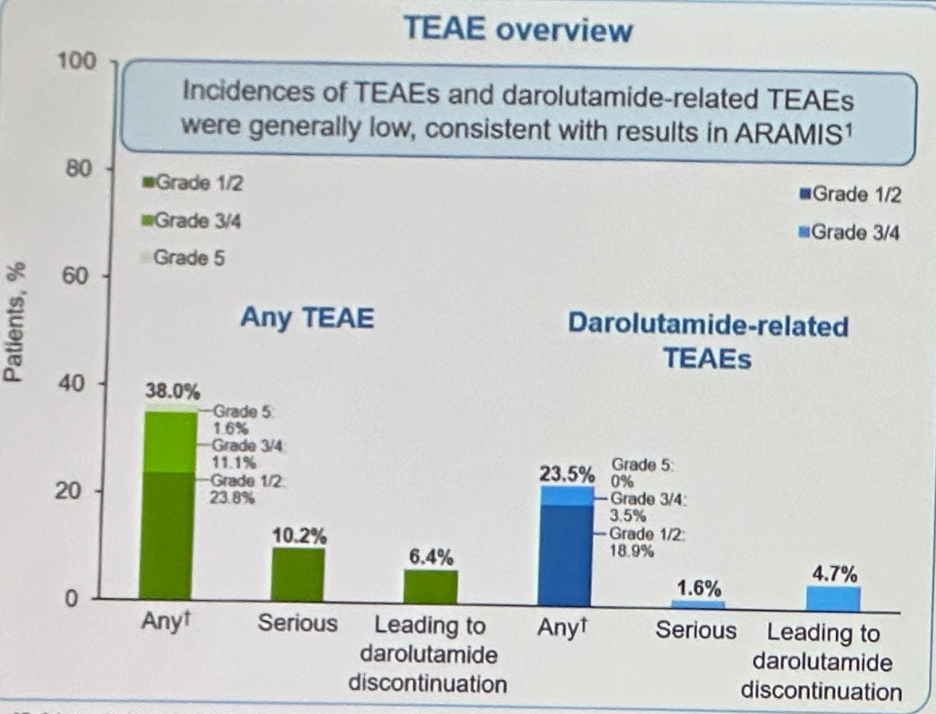
The median follow-up at this interim analysis was 16.5 months (IQR: 12.5 – 23.1). The median treatment duration was 14.9 months (1QR 10.4-20.9) The incidences of treatment-emergent (TEAEs) and darolutamide-related adverse events were generally low, consistent with the results in ARAMIS. Any grade TEAE occurred in 38% of patients, with serious events and those leading to darolutamide discontinuation occurring in 10.2% and 6.4% of patients, respectively. Darolutamide-related TEAEs occurred in 23.5% of patients, with serious events and those leading to darolutamide discontinuation occurring in 1.6% and 4.7% of patients, respectively.
The most common TEAEs were fatigue, rash, and falls, as summarized below:

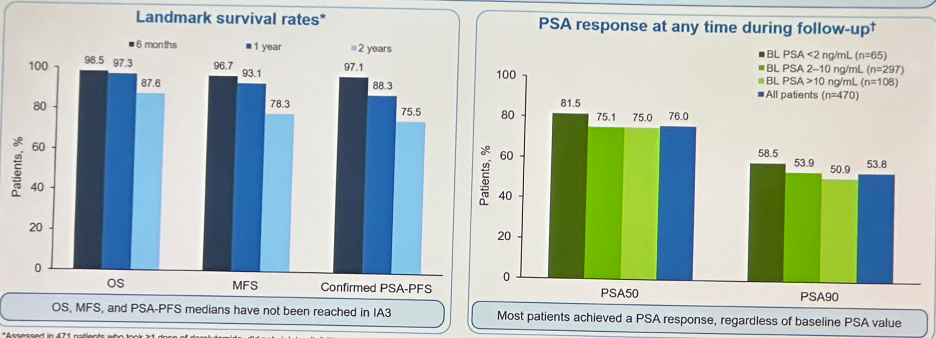
With regards to efficacy outcomes, metastasis-free survival, overall survival, and PSA outcomes indicate effectiveness in the real-world setting, consistent with ARAMIS.1,2 At 2 years follow-up, 88% of patients were alive and 78.3% were free of metastases. The median overall, metastasis-free, and PSA progression-free survivals have not yet been reached.
Dr. Gotto concluded that under real-world conditions, darolutamide shows a favorable safety profile in nmCRPC patients, consistent with those observed in ARAMIS. The incidence of TEAEs were generally low, with no new safety signals during this interim analysis. The metastasis-free survival, overall survival, and PSA outcomes from this analysis indicate the real-world effectiveness of darolutamide in patients with nmCRPC, consistent with ARAMIS. Further analyses with more patients and longer follow-ups are planned, which will provide mature effectiveness outcomes.
Presented by: Geoffrey Gotto, MD, MPH, FRCSC, Clinical Associate Professor, Department of Surgery, University of Calgary, Calgary, Alberta
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024
References:
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020 Sep 10;383(11):1040-1049.


