(UroToday.com) During the American Urological Association’s annual meeting, Dr. Hannah Kay presented research assessing the readability of various QoL questionnaires used in urologic oncology. She began by explaining the importance of effectively assessing the quality of life (QoL) of patients with cancer, reviewing that this process heavily relies on patients' ability to self-report their experiences through questionnaires.
However, the readability of these instruments is crucial, as it influences how well patients can comprehend and thus accurately convey their health status. This study aimed to determine whether these questionnaires adhere to the American Medical Association (AMA) and National Institutes of Health (NIH) recommendations, which suggest that health information should be written at a 6th-grade reading level or lower to ensure it is understandable to the vast majority of patients.
Dr. Kay continued that the study evaluated five European Organisation for Research and Treatment of Cancer (EORTC) questionnaires specific to genitourinary (GU) cancers: QLQ-C30 for general cancer patients, QLQ-BLM30 for muscle-invasive bladder cancer (MIBC), QLQ-NMIBC24 for non-muscle invasive bladder cancer (NMIBC), QLQ-PR25 for prostate cancer, and QLQ-TC26 for testicular cancer. These instruments were assessed using several readability metrics, including the Automated Readability Index (ARI), Flesch Reading Ease (FRE), Flesch-Kincaid Grade Level (FKGL), Simple Measure of Gobbledygook (SMOG), and Linsear Write Formula (LWF). The findings revealed that only the QLQ-C30 and QLQ-TC26 questionnaires meet the AMA and NIH readability standards, being comprehensible at a 6th-grade reading level. The other questionnaires were found to be written at a higher grade level, potentially complicating the understanding for a significant portion of the patient population.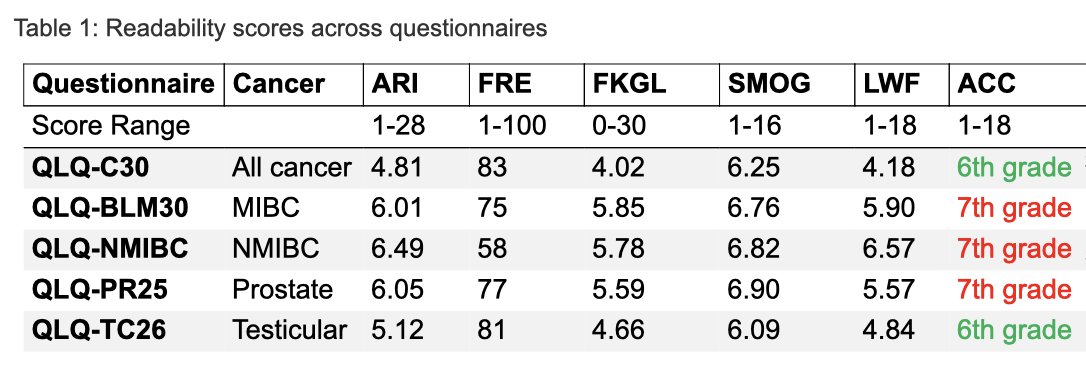
Next, Dr. Kay highlighted the need for ongoing revisions and considerations in the development of QoL assessments used in clinical settings, particularly in oncology. She concluded by emphasizing that these instruments must be accessible and understandable to all patients in order to gather accurate data on patient outcomes, which in turn can guide more tailored and effective treatment plans.
Presented by: Hannah Kay, MD, UNC Chapel Hill, Chapel Hill, NC
Written by: Ruchika Talwar, MD, Urologic Oncology Fellow, Vanderbilt University Medical Center, during the 2024 AUA Annual Meeting, San Antonio, TX, May 3rd to May 6th


