(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on prostate cancer trials in progress, and a presentation by Dr. Neeraj Agarwal discussing EvoPAR-Prostate01, a phase III, double-blind, placebo-controlled, 2-cohort, randomized study of saruparib (AZD5305) in combination with new hormonal agents in patients with metastatic castration-sensitive prostate cancer (mCSPC) with and without homologous recombination repair (HRR) mutations. ADT plus new hormonal agents have improved outcomes for patients with mCSPC, but patients will eventually progress to mCRPC, which is associated with poor survival outcomes. As such, there is a need for effective treatments in mCSPC that can delay initiation of chemotherapy and progression to mCRPC. Combinations of PARP inhibitor plus new hormonal agents have demonstrated clinical benefit in patients with mCRPC [1-3]. In other indications, the clinical activity of PARP inhibitors in earlier lines of treatment has demonstrated potential to provide greater magnitude of benefit and delay disease progression. Of note, the efficacy and safety of PARP inhibitor therapy for patients with HRR mutated mCSPC are being assessed in the ongoing phase III studies TALAPRO-3 and AMPLITUDE.
Saruparib (AZD5305), a first-in-class PARP1 inhibitor, was developed through rational design to be highly selective for PARP1, with increased potency and improved physicochemical properties versus other approved PARP inhibitors. In the PETRA study (NCT04644068), the favorable safety profile and low dose-reduction rate observed with saruparib monotherapy compared with approved PARP inhibitors suggest that patients may be able to remain on treatment longer at an optimal dose (60 mg QD), which may improve efficacy. The safety and efficacy of saruparib plus new hormonal agents for the treatment of mCSPC and mCRPC are being assessed in the Phase I/IIa PETRANHA study (NCT05367440), with the initial data indicated that saruparib (60 mg QD) can be safely combined with enzalutamide, abiraterone acetate, or darolutamide. Low rates of hematologic and gastrointestinal toxicities, as well as low rates of dose reductions or discontinuations, were observed.
EvoPAR-Prostate01 will assess a mCSPC patient’s HRR mutation biomarker status, at which time they will enter the HRR mutation (~550 patients) or the non-HRR mutation (~1,250) cohort of the trial, followed by randomization 1:1 to either saruparib 60 mg plus physician’s choice of new hormonal agent versus placebo plus physician’s choice of new hormonal agent. The trial design is as follows:
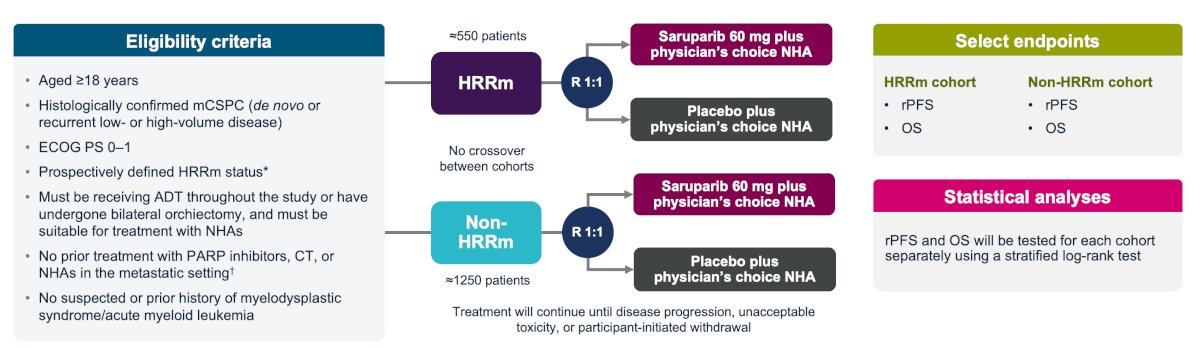
The selected endpoints for both cohorts will be radiographic progression free survival and overall survival. Accrual began in November 2023 and is ongoing. There are 370 study sites that are recruiting or planning to recruit patients from 26 countries across Asia-Pacific, Europe, North America, and South America:
Presented by: Neeraj Agarwal, MD, FASCO, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, May 3-6, 2024, San Antonio, Texas
References:
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043.


