(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX was host to a bladder and upper tract transitional cell carcinoma podium session. Dr. Ronald Kaufman presented the preliminary results of a phase 3 trial evaluating the efficacy and safety of padeliporfin vascular targeted photodynamic therapy for the treatment of low-grade upper tract urothelial carcinoma.
Upper tract urothelial carcinoma accounts for only 5–10% of urothelial cancers. The annual incidence in the USA is ~2 cases per 100,000 population. Up to 60% of such tumors occur in the pyelocaliceal system and 40% in the ureter. The peak incidence is between 70 and 90 years of age. This disease occurs three-fold more commonly in men. Known risk factors include smoking, alcohol consumption, aristolochic acid, and Lynch syndrome.
Chronic kidney disease remains a clinical issue of utmost significance encountered in the management of patients with upper tract urothelial carcinoma. Tumor ablation is the recommended initial management option for patients with favorable, low-risk upper tract urothelial carcinoma. Tumor ablation may also be offered to patients with unfavorable, low-risk upper tract urothelial carcinoma and select patients with favorable, high-risk disease who have low-volume tumors or cannot undergo a radical nephroureterectomy. Endoscopic surveillance is critical in these patients, and effective organ-sparing options in this population remain an unmet clinical need.
The ENdoluminal LIGHT activated treatment of upper tract urothelial carcinoma (ENLIGHTED; NCT 04620239) trial is a single arm, open label, global pivotal phase III trial being conducted across 29 sites in the United States, France, Spain, Italy, Germany, Austria, and Israel. The target sample size is 100 patient enrollees, of whom 75 are likely to be evaluable.
Padeliporfin vascular targeted photodynamic therapy (VTP) is a combination product, whereby the drug, padeliporfin (a photosensitizer), is intravenously administered and a device, a laser light delivery system, includes a source of light (the laser) that emits near-infrared light at 753 nm, and optic fiber that delivers the light to the target lesion(s) in the upper tract urothelium.

Upon light activation, Padeliporfin triggers a cascade of pathophysiological events that have a strong impact on tumor vasculature, and consists of the following consecutive steps:

The study hypothesis was that Padeliporfin VTP treatment would provide effective complete ablation of low-grade upper tract urothelial carcinoma lesions that will be durably maintained to support the ultimate clinical aim of kidney preservation. The primary objective was to demonstrate the efficacy and durability of effect following padeliporfin VTP on low grade upper tract urothelial carcinoma tumors in the kidney and ureter at Primary Response Evaluation (PRE) during padeliporfin VTP Induction Treatment Phase. Secondary objectives were to evaluate Padeliporfin VTP-related safety and tolerability in the treatment of low-grade upper tract urothelial carcinoma tumors in the kidney and ureter.
This study included patients with new or recurrent low-grade, non-invasive upper tract urothelial carcinoma, meeting the following criteria:
- Up to 2 biopsy-proven tumor lesions of low-grade involvement with the largest index tumor 5-15 mm in diameter (as measured by endoscopy), located in the calyces, renal pelvis, or in the ureter of the ipsilateral kidney, with an absence of high-grade cells on cytology. (Ureter involvement can be in one ureter location with no more than 120 mm of contiguous ureteral length)
Key exclusion criteria included:
- Current high-grade or muscle invasive (>pT1) urothelial carcinoma of the bladder
- Carcinoma in situ (CIS) – current or previous in the upper urinary tract
- History of invasive T2 or higher urothelial cancer in the preceding two years
- Prohibited medication that could not be adjusted or discontinued prior to study treatment
- Patients with photosensitive skin diseases or porphyria
The study schema is illustrated below. Patients underwent initial screening to confirm eligibility. They subsequently underwent induction treatment at 1–3 months thereafter. They received 1–3 padeliporfin VTP treatments under anesthesia about four weeks apart. If a complete response (defined as no visible tumor endoscopically, negative biopsy/cytology) was not achieved after three treatments on Visit 2 (or if the disease had progressed), patients were discontinued from the Treatment Phases and entered the long-term follow-up phase.
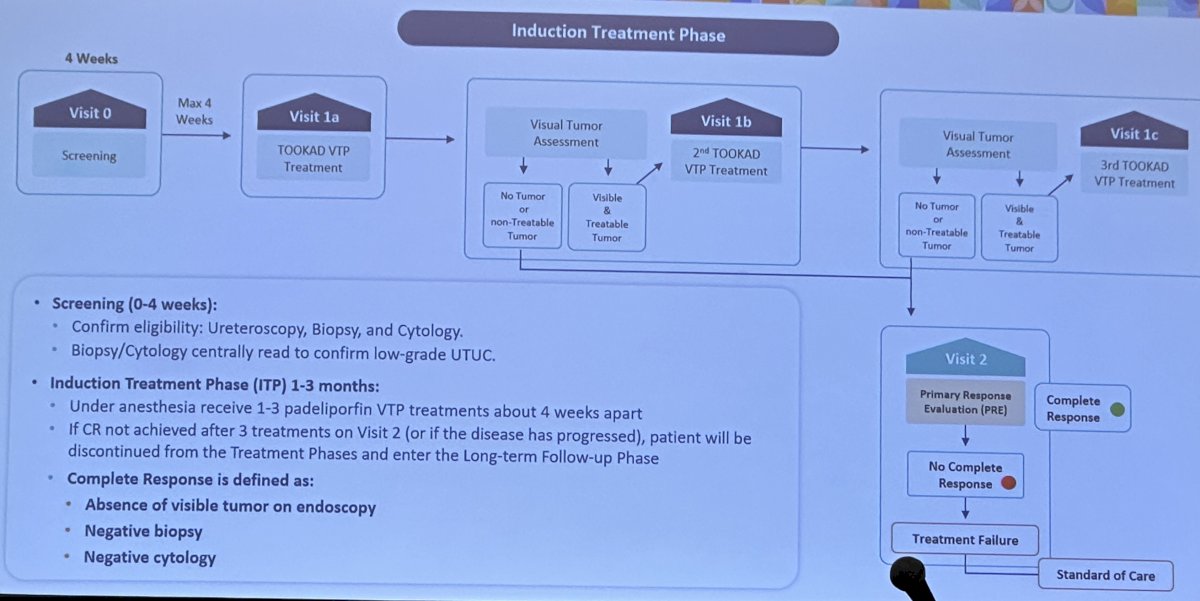
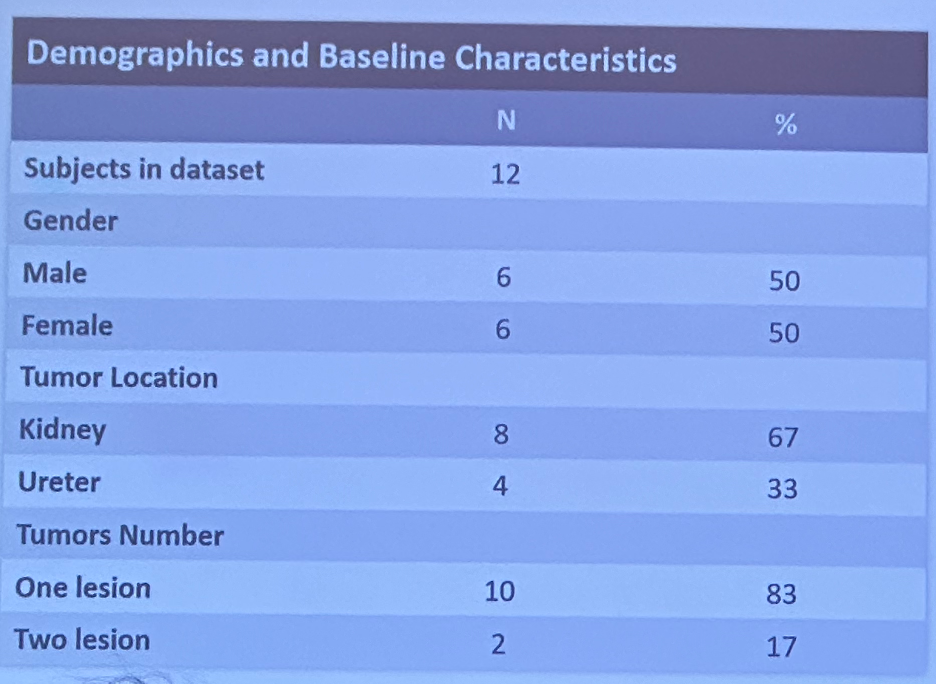
At presentation, 12 patients had been treated, and nine had completed Visit 2. Of these 9 patients, 6 (675) achieved a complete response and 3 (33%) achieved a partial response.
With regards to adverse events, the majority were grade 1–2 in severity, with grade 3 serious adverse events observed in 6% of patients (flank pain, hypertension, renal colic). All adverse events resolved within 2-3 days. No grade 4–5 adverse events were observed.
Based on these preliminary results, Dr. Kaufman concluded that:
- Padeliporfin VTP demonstrates early evidence of safety and efficacy
- Recruitment for the ENLIGHTED trial is ongoing
- Long-term results are expected to provide the basis for treatment approval
Presented by: Ronald Kaufman Jr., MD, Professor, Department of Urology, Albany Med Health System, Albany, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.


