(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Upper Tract Transitional Cell Carcinoma podium session. Dr. Yair Lotan presented the results of a large multicentric study of patients with upper tract urothelial carcinoma treated with UGN-101.
Dr. Lotan opened his presentation by talking about UGN-101, a novel therapeutic agent for the treatment of upper tract urothelial carcinoma (UTUC) and bladder cancer. UGN-101 (Jelmyto™) is a novel formulation of mitomycin C that uses a unique hydrogel designed to increase urinary dwell time. He discussed the OLYMPUS trial inclusion criteria for chemoablation: Low-grade 5-15mm pelvicalyceal tumors.1
He discussed the OLYMPUS trial showed a complete response (CR) rate of 59%. However, this was a small trial (n=71), with limited follow-up (median 11 months) and there is no “real-world” data using UGN-101 reported to date. The Kaplan-Meier plot below shows the proportion of patients with durable CR in the OLYMPUS trial with a median follow-up of 12 months.2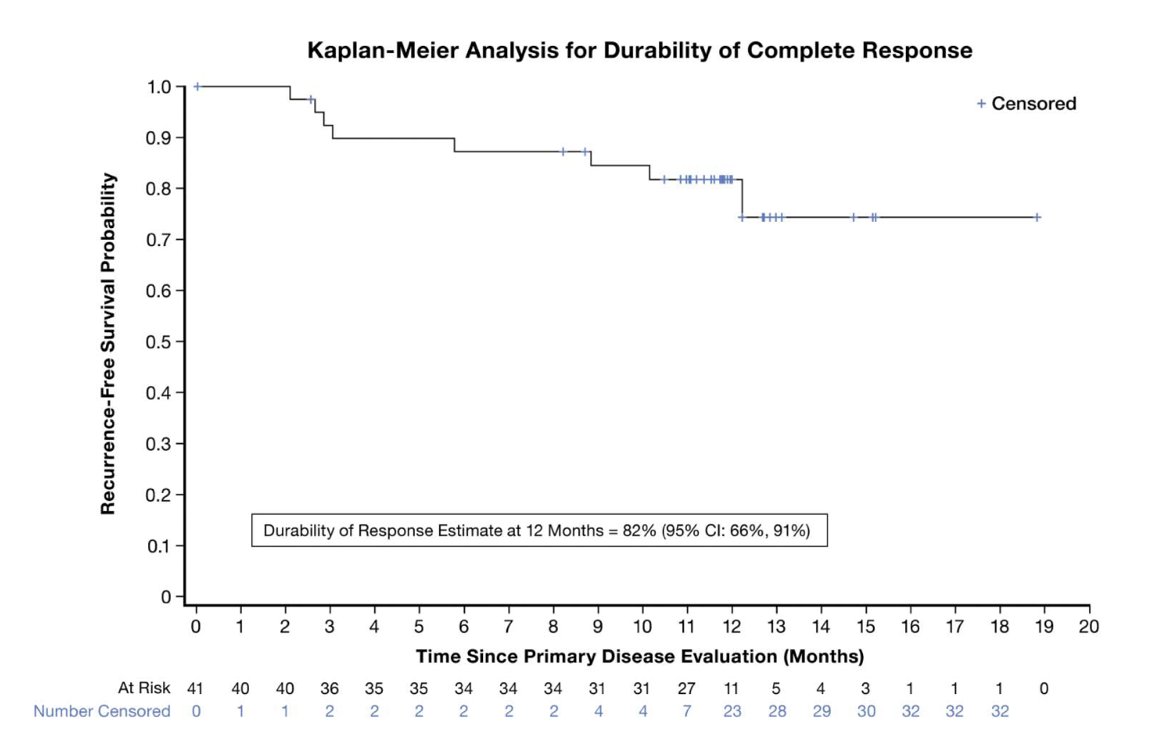
Dr. Lotan noted that their study evaluated a large multicenter cohort of patients from 15 different centers in the U.S. treated with UGN-101 for UTUC.
This analysis included 136 renal units the co-primary outcomes were recurrence-free survival (RFS), and this was calculated only for patients who had no evidence of disease following UGN-101 induction and progression-free survival (PFS) was calculated for all patients treated with UGN-101. Disease progression was defined as:
- Grade progression from low-grade to high-grade disease,
- Stage progression
- Development of metastatic disease.
The inclusion criteria of this study reflected more the real-world experience with UGN-101 and they allowed for:
- Chemoablation of larger tumors
- Adjuvant intent UGN-101
- Ureteral tumors
- High-grade disease
Dr. Lotan presented data on 136 renal units treated with UGN-101. Among these patients, the majority were male (70%), predominantly white (83%), and had a history of smoking (78%). Most had experienced recurrence following prior intracavitary treatment, and only 9% had ureteral involvement. Additionally, 50% of the renal units had multifocal disease, 43% had the tumor cleared endoscopically before UGN-101 treatment, and 9% had high-grade disease.
When examining the response to UGN-101 in patients based on tumor burden prior to induction, the investigators noted the best response was observed in patients with completely ablated disease, with 69.2% (n=36) achieving complete response. Similarly, patients with tumors < 1cm at the time of induction had a complete response rate of 70% (n=7). However, patients with tumors measuring 1-3 cm or >3 cm demonstrated lower rates of complete response, at 33.3% and 23.5%, respectively. 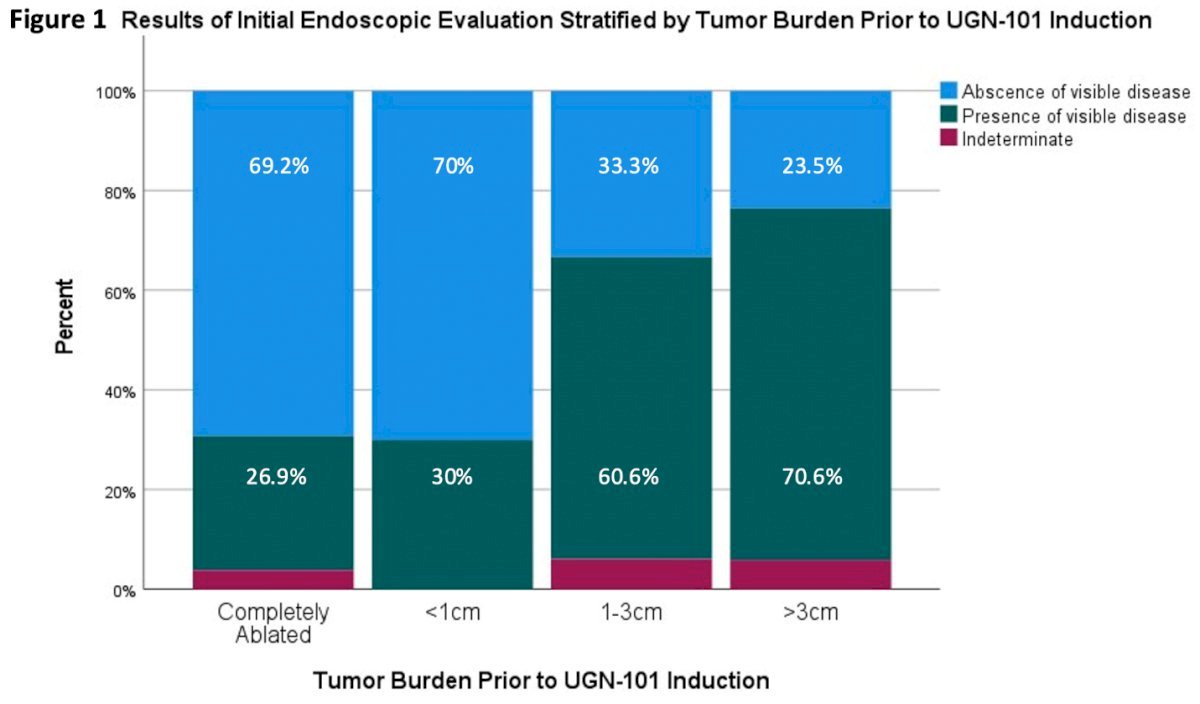
When looking at the response of residual disease in the following UGN-101 for chemoablation cohort, 59 patients were included in the analysis, of these 23 had ureteral tumors and 36 pelvicalyceal tumors. Among the 23 patients, 48% achieved a CR, 26% a partial response, and 26% no response. Similar to the pelvicalyceal tumor cohort, 31% achieved a CR, 50% a partial response and 19% had no response.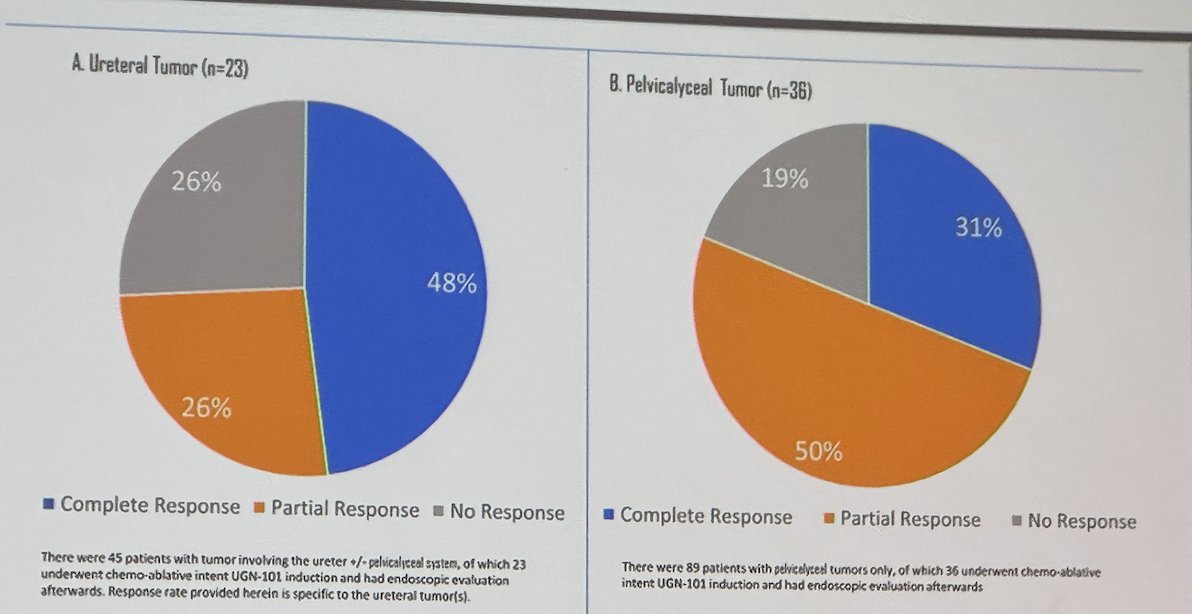
In patients with low-grade UTUC treated with UGN-101 the median time to recurrence was not reached. Figure below: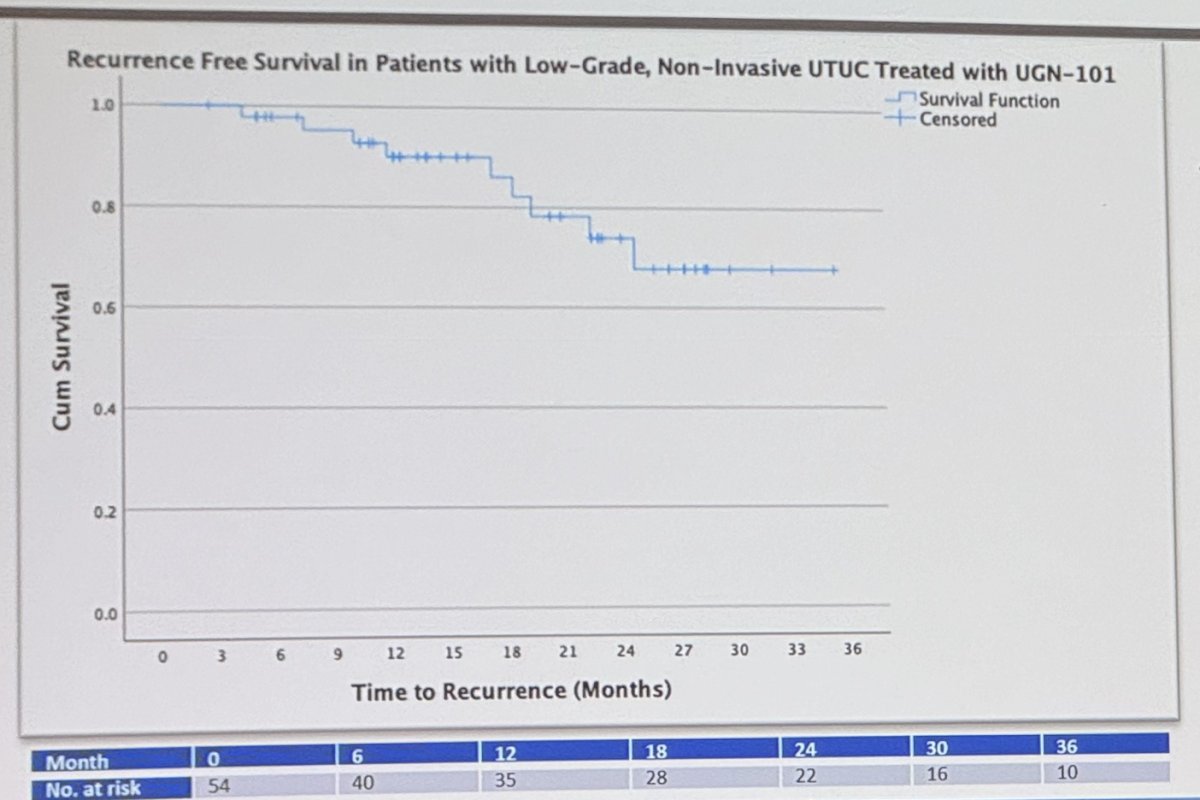
Among initial responders to induction, 30% received maintenance therapy (n=13), and the RFS at 24 months was 100% for those who received maintenance compared to 61% for those who did not (p=0.014). However, there is no evidence for using maintenance besides this real-world data, to date.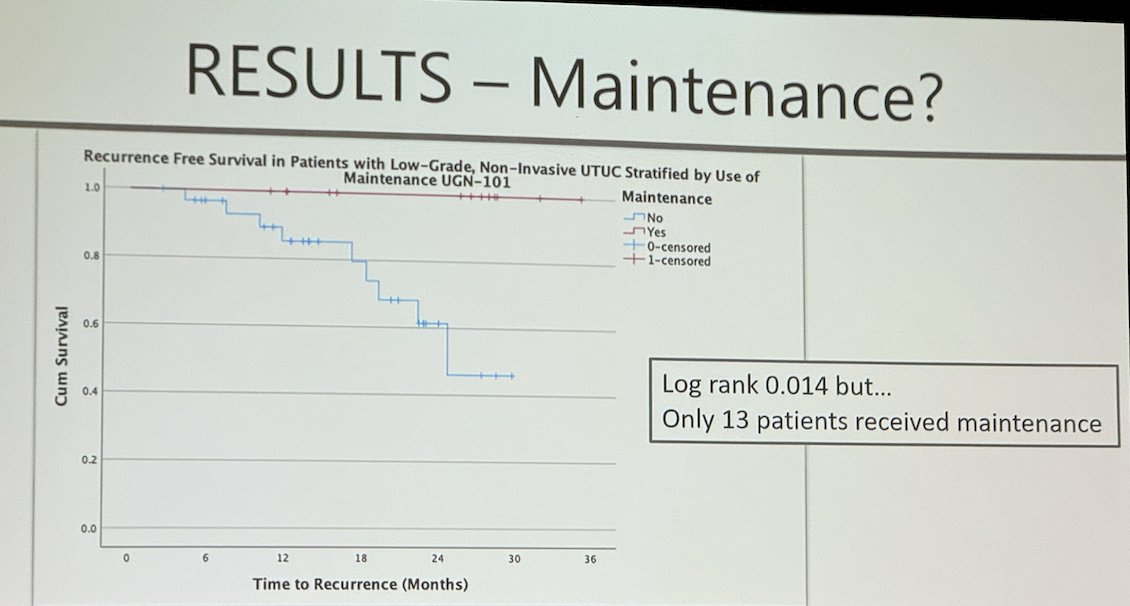
They reported that 9 cases of high-grade and presumed non-invasive disease treated with UGN-101 showed a median risk of progression of 50% at 12 months. In the subset of 4 cases with HGTa disease who had no evidence of disease following UGN-101 induction, 50% had recurred by 10 months. 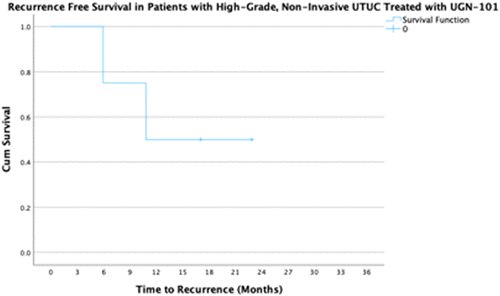
Dr. Lotan concluded his presentation by emphasizing that in real-world practice, the approach differed from the registration trial in various clinical contexts, including tumors below the UPJ, after complete endoscopic ablation, in tumors >1.5cm, and occasionally for high-grade histology.
He affirmed that, according to their data, UGN-101 treatment demonstrates overall good durability of response among initial responders and a low rate of progression. He emphasized the need for a larger cohort to determine if there are other individual factors predictive of recurrence. Additionally, more data are required to establish the benefit of maintenance therapy, and he suggested that a prospective registry could help address these uncertainties.
Presented by: Yair Lotan, MD. Urologist, UT Southwestern Medical Center, Dallas, TX
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
References:
- Kleinmann N, Matin SF, Pierorazio PM, Gore JL, Shabsigh A, Hu B, Chamie K, Godoy G, Hubosky S, Rivera M, O'Donnell M, Quek M, Raman JD, Knoedler JJ, Scherr D, Stern J, Weight C, Weizer A, Woods M, Kaimakliotis H, Smith AB, Linehan J, Coleman J, Humphreys MR, Pak R, Lifshitz D, Verni M, Adibi M, Amin MB, Seltzer E, Klein I, Konorty M, Strauss-Ayali D, Hakim G, Schoenberg M, Lerner SP. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020 Jun;21(6):776-785. doi: 10.1016/S1470-2045(20)30147-9. Epub 2020 Apr 29. PMID: 32631491.
- Matin SF, Pierorazio PM, Kleinmann N, Gore JL, Shabsigh A, Hu B, Chamie K, Godoy G, Hubosky SG, Rivera M, O'Donnell M, Quek M, Raman JD, Knoedler JJ, Scherr D, Weight C, Weizer A, Woods M, Kaimakliotis H, Smith AB, Linehan J, Coleman J, Humphreys MR, Pak R, Lifshitz D, Verni M, Klein I, Konorty M, Strauss-Ayali D, Hakim G, Seltzer E, Schoenberg M, Lerner SP. Durability of Response to Primary Chemoablation of Low-Grade Upper Tract Urothelial Carcinoma Using UGN-101, a Mitomycin-Containing Reverse Thermal Gel: OLYMPUS Trial Final Report. J Urol. 2022 Apr;207(4):779-788. doi: 10.1097/JU.0000000000002350. Epub 2021 Dec 17. PMID: 34915741.


