(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Upper Tract Transitional Cell Carcinoma podium session. Ian McElree presented the results of a retrospective study comparing sequential endoluminal gemcitabine plus docetaxel versus Bacillus Calmette-Guerin (BCG) for the treatment of upper tract urothelial carcinoma in situ (CIS).
The investigator began his presentation by describing that upper tract urothelial carcinoma represents less than 10% of urothelial cancers and is usually associated with poorer oncological outcomes (30% metastatic at diagnosis). Currently, BCG remains the only approved endoluminal treatment option for the management of high-grade UTUC after complete tumor ablation of disease and is endorsed by the AUA/SUO guidelines. The combination of endoluminal gemcitabine + docetaxel has shown efficacy for the management of BCG unresponsive non-muscle invasive bladder cancer with an impressive high-grade recurrence-free survival (HR-RFS) of 84% at 2 years,1 and high-grade UTUC (Table below).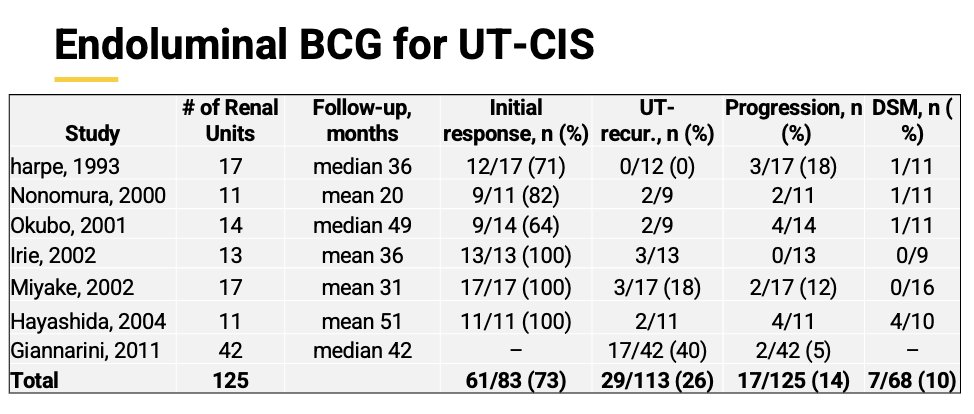
A direct comparison of this combination with BCG has not been made to date. The primary objective of this study was to compare the outcomes of high-grade UTUC patients receiving endoluminal gemcitabine/cisplatin vs. patients receiving endoluminal BCG.
This retrospective analysis included 53 patients, with 65 upper tract tumors, of which 34 and 31 were treated with gemcitabine/docetaxel and BCG, respectively. Patients had the following treatment schedules:
Induction- Gemcitabine/docetaxel group: Six weekly induction instillations of 1 gm gemcitabine + 37.5 mg docetaxel instilled via a percutaneous nephrostomy tube or a retrograde ureteral catheter.
- BCG group: Received 1 vial of BCG (+/- IFNa-2b) Six weekly induction instillations via percutaneous nephrostomy tube or a retrograde ureteral catheter.
- Gemcitabine/Docetaxel group: Monthly for 6 months
- BCG group: A single 3-week mini-cycle
The primary outcome was recurrence-free survival (RFS). Survival analyses using Kaplan-Meier estimates and Cox regression modeling were performed. Baseline patient characteristics showed most patients didn’t have a history of UTUC (77% BCG and 85% Gem/Doce). 65% of patients in the BCG group were treated with a retrograde catheter compared to 68% in the Gem/Doce group. Other baseline characteristics are shown in the table below: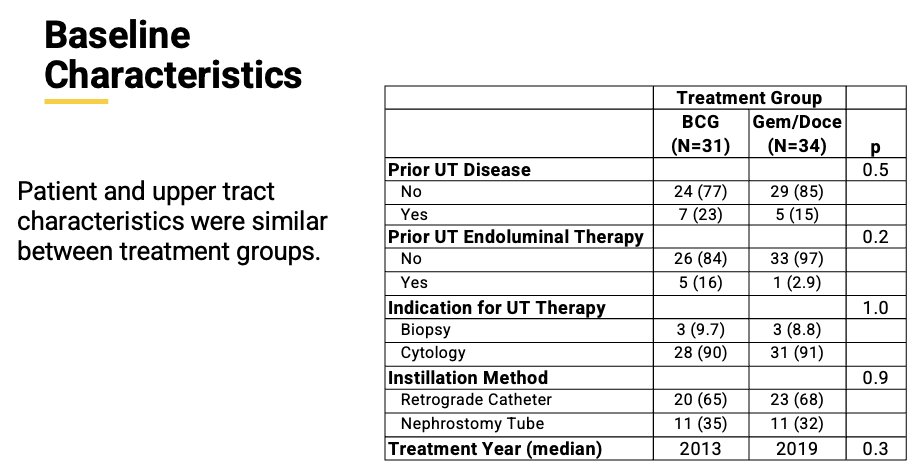
The median follow-up for BCG-treated patients was 58 months and 27 months for gemcitabine/docetaxel treated patients. The 2-year HG-RFS was similar in the two treatment groups: 61% for BCG and 54% for gemcitabine/docetaxel.

The investigators found, through univariate analysis, that employing a nephrostomy tube for antegrade instillation of either BCG or Gem/Doce was linked to over a threefold increase in the risk of recurrence compared to retrograde instillation with a ureteral catheter (HR 3.89, 95% CI 1.59-9.53, p<0.01).
The nephroureterectomy-free survival at 2 years was 89% with BCG and 100% with gemcitabine/docetaxel. Similarly, the progression-free survival at 2 years was 84% for BCG vs. 93% for Gem/Doce.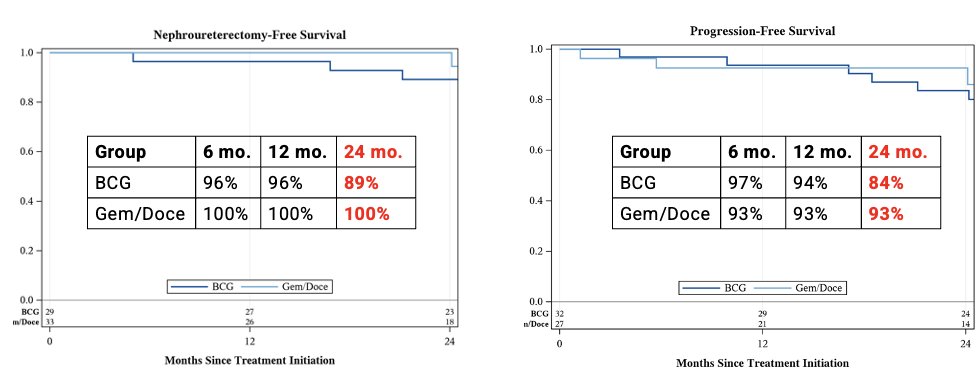
The investigators showed that in their study, there were similar rates of adverse events comparing gemcitabine/docetaxel vs. BCG (p=0.12). In total, 11% of patients receiving BCG and 7.7% of patients receiving Gemcitabine/docetaxel experienced a grade 3+ adverse event.
Ian McElree ended his presentation concluding that endoluminal gemcitabine/docetaxel and BCG have similar oncological outcomes and similar rates of adverse events for the treatment of high-grade UTUC. Although the results from this retrospective study support the use of gemcitabine/docetaxel as an alternative to BCG, further prospective evaluation is warranted for new recommendations to be made.
Presented by: Ian McElree, Medical Student (MS3), The University of Iowa Carver College of Medicine, Iowa City, Iowa City, IA
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
References:


