(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Upper Tract Transitional Cell Carcinoma podium session. Dr. Adam Feldman presented the results of a multicentre study of UGN-101 for the treatment of upper tract urothelial cancer (UTUC).
Dr. Feldman commenced his presentation by showing how UGN-101 which was Originally approved for chemoablative treatment in cases of low-volume, low-grade disease within the pelvicalyceal system based on the findings of the OLYMPUS study,1 is now being increasingly utilized in the “real world” for a wider range of applications, including bulky tumors, adjuvant therapy, and cases involving the ureter.
The investigators shared that data presented from this study was gathered from 15 medical centers on patients who underwent treatment with UGN-101 for UTUC. Recurrence-free survival (RFS) was computed exclusively for individuals who exhibited no signs of disease after UGN-101 induction. They clarified the definitions used in the study:
- Chemoablation: referred to the administration of UGN-101 induction in cases where residual UTUC was known.
- Adjuvant use: denoted the administration of UGN-101 after visually complete endoscopic ablation.
Exploratory analyses were conducted to evaluate the impact of tumor size, tumor location (renal pelvis vs ureter vs. both), multifocality, and instillation technique (antegrade vs. retrograde).
The investigators analyzed 136 renal units with UTUC treated in 132 patients. The cumulative median follow-up was 22 (IQR 12-27) months. The cohort included 118 renal units with low-grade presumably non-invasive UTUC. He mentioned that 100 renal units had endoscopic evaluation following induction therapy.
Upon initial evaluation, 74% of patients receiving adjuvant treatment and 39% of those undergoing chemoablative therapy were found to be disease-free, totaling 54 cases of UTUC showing no evidence of disease post-UGN-101 induction. The status prior to UGN-101 induction was no evidence of disease and received Adjuvant therapy (59.3%), residual disease, and received Chemoablative (40.7%). Approximately 44% had multifocal disease, the tumor was located in the pelvis in 61%, ureter in 15% and both in 24%. The instillation technique was retrograde in 50% and antegrade in 50%.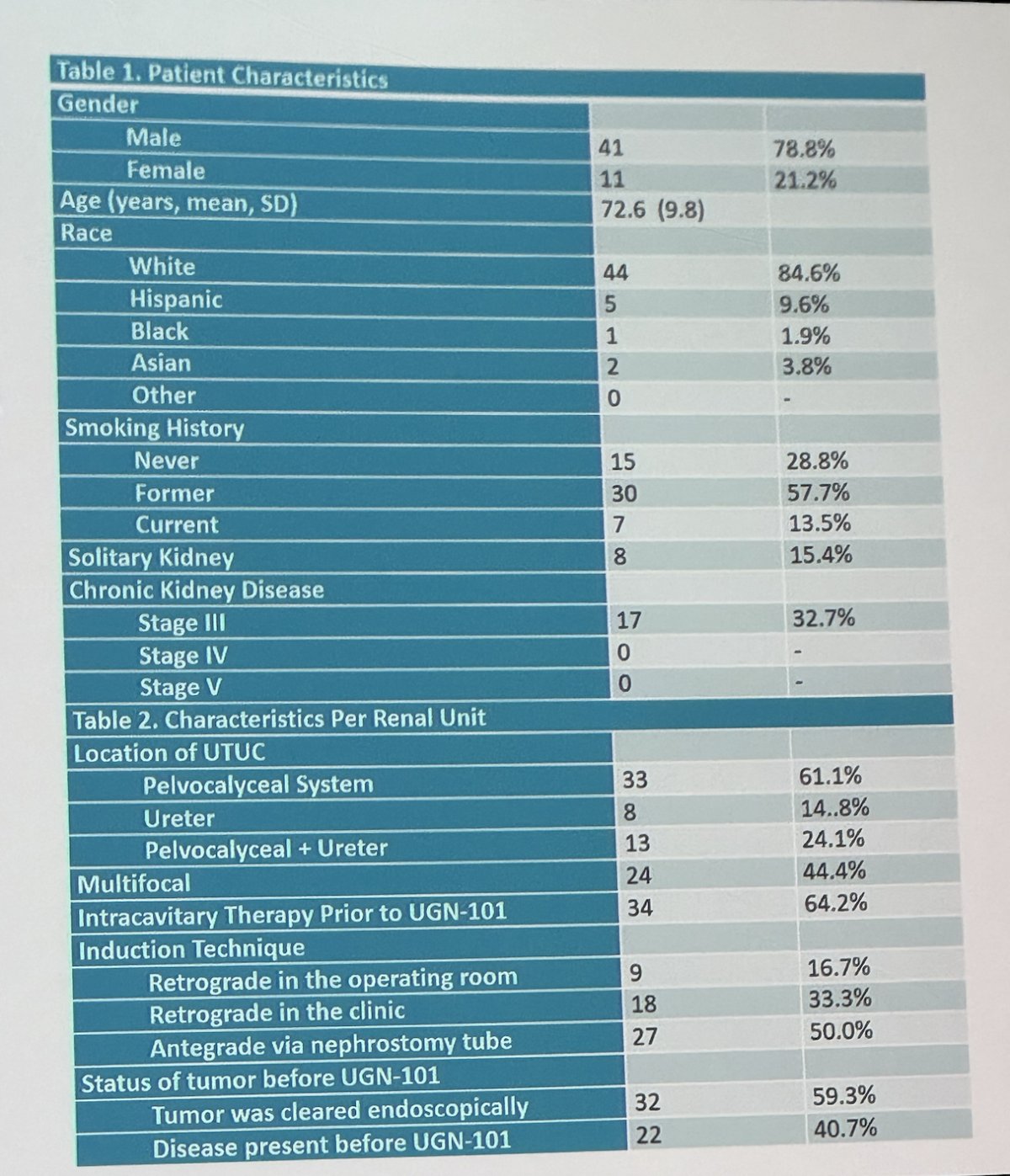
The overall recurrence-free survival (RFS) rate was 68% % at 3 years. The Kaplan-Meier curve is shown below.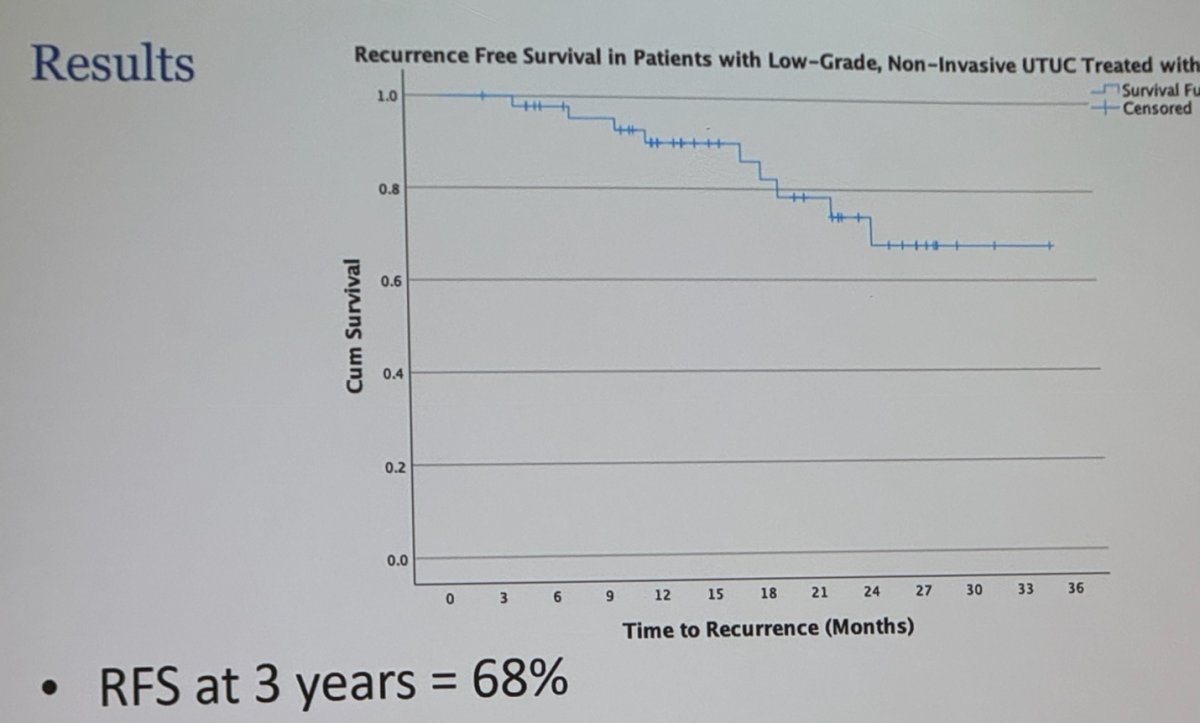
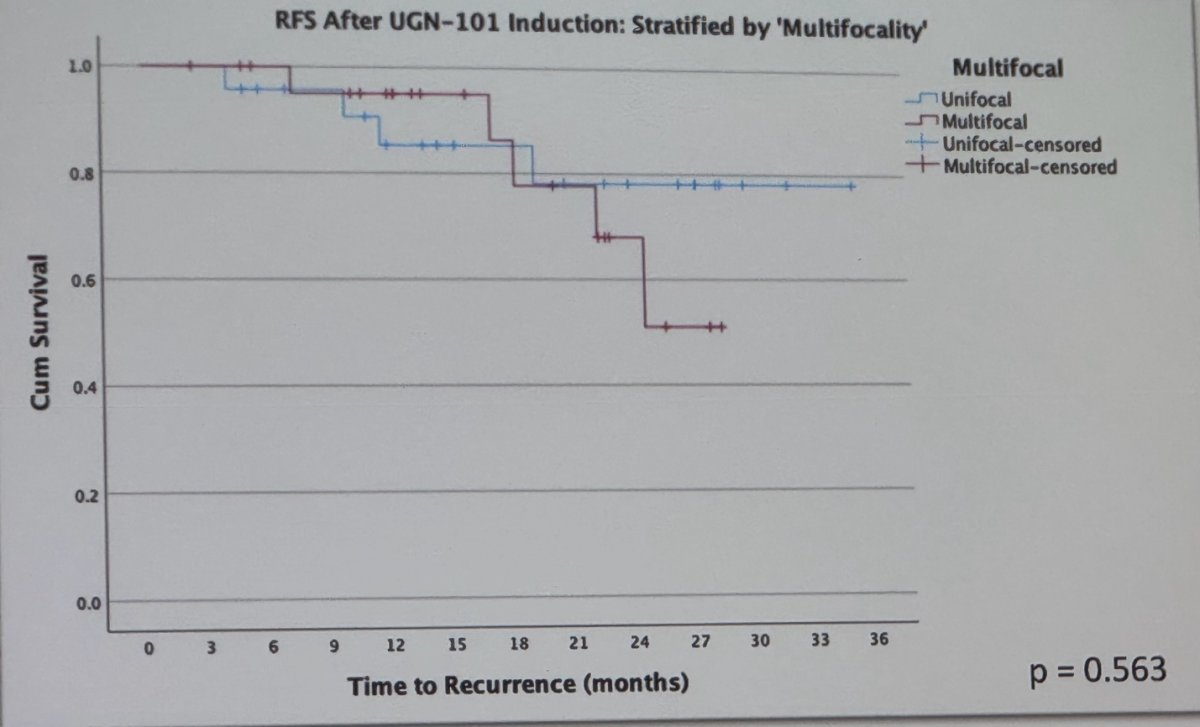
Among these patients, there was no discernible difference in RFS based on initial disease characteristics size, location (p=0.455) multifocality (p=0.563), or treatment approach (chemoablative vs. adjuvant intent of UGN-101 induction) (p=0.597). The Kaplan Meier plots are shown below.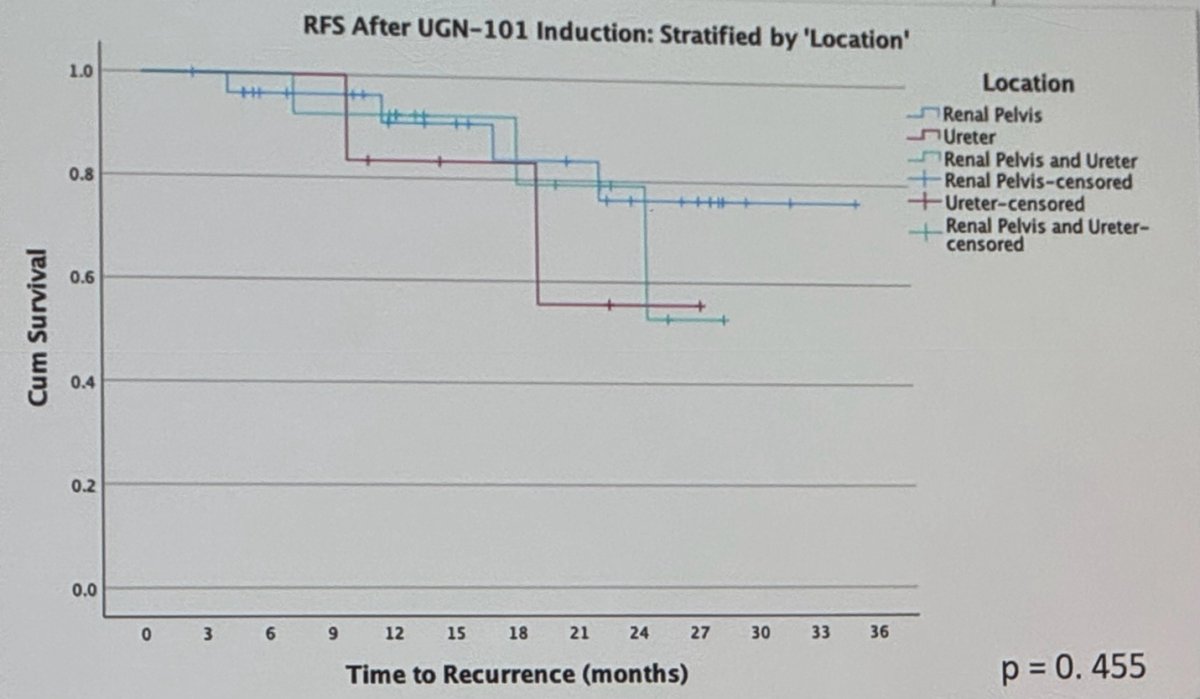
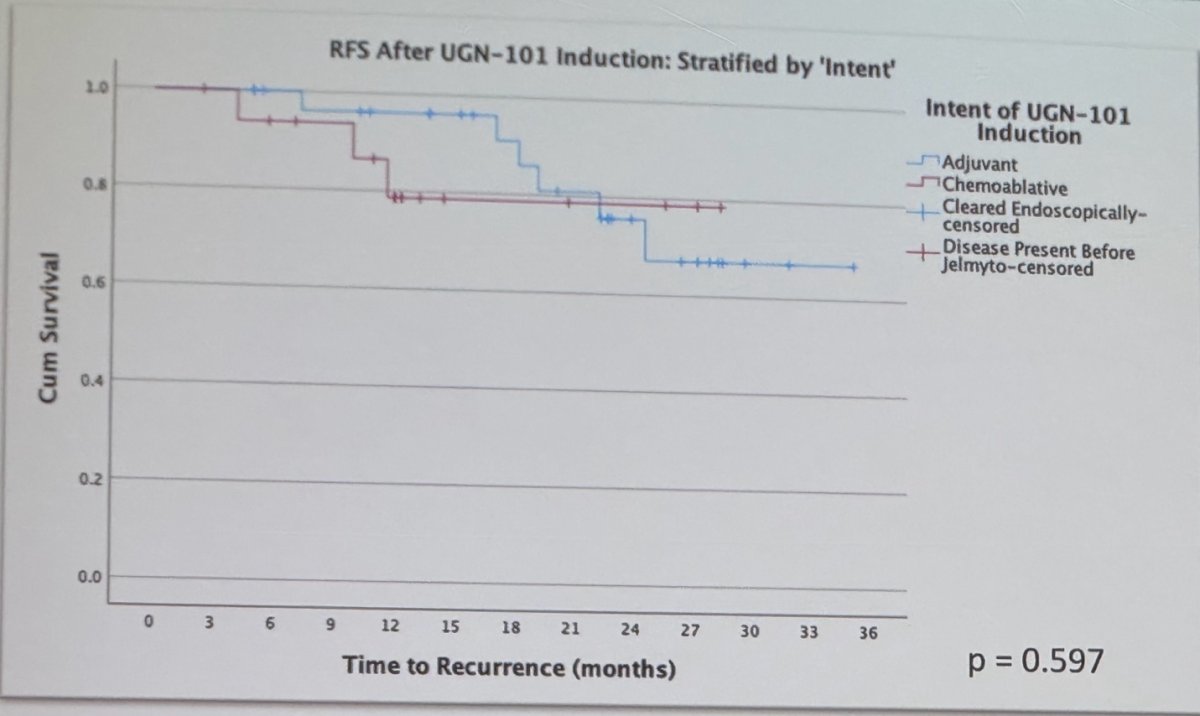
Dr. Feldman concluded his presentation by affirming that UGN-101 treatment shows promising recurrence-free survival rates for patients with LG presumed non-invasive UTUC who respond positively to initial induction. Furthermore, there seems to be no difference in recurrence rates based on the intent of UGN-101 induction (whether adjuvant or chemoablative), original tumor size, multifocality, or tumor location.
Presented by: Adam Feldman, MD. Urologic Oncologist, Massachusetts General Hospital, Boston, MA
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
Reference:


