(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Society of Urologic Oncology (SUO) session Dr. Hooman Djaladat debated in favor of doing a lymph node dissection for Upper Tract urothelial carcinoma (UTUC).
Dr. Djaladat highlighted the uncertainty surrounding lymph node dissection (LND) for UTUC due to the lack of good randomized controlled data supporting its use. He mentioned ongoing trials aimed at addressing this clinical dilemma, including one in Shanghai, China (NCT03544437) assessing the feasibility and safety of extraperitoneal laparoscopic extended retroperitoneal lymph node dissection during nephroureterectomy. Additionally, there is a trial in Cleveland, Ohio (NCT06262516) focusing on lymph node dissection for UTUC (not yet recruiting), along with two more trials in Beijing, China (NCT03474926 and NCT03230201).
Dr. Djaladat organized his talk into four parts: the benefits of LND in UTUC, the harms of LND, the indications for LND, and recommendations for LND for upper tract disease in 2024. He presented a case of a 79-year-old man with gross hematuria and an ill-defined lobulated mass occupying the majority of the right renal collecting system and extending into the proximal right ureter. This patient had a negative metastatic work-up and no evidence of disease in the bladder. Ureteroscopic biopsy revealed non-invasive low-grade UTUC, with concern for high-grade disease. Dr. Djaladat and Dr. Pierorazio agreed that they would have performed an LND in this case.
He discussed the rising incidence of UTUC over the past two decades, with increases seen in UTUC in the ureter, pyelocaliceal system, and overall UTUC. This data was presented graphically.
Dr. Djaladat discussed that regional lymph node metastases are present in up to one-third of UTUC patients at the time of diagnosis, and lymph node involvement increases with higher pathological T stages. The graphic below illustrates how lymph node involvement risk is almost 80% for pT4, over 20% for pT3, and less than 10% for pT2. He posed the question of whether this data alone should convince us to perform an LND for UTUC disease.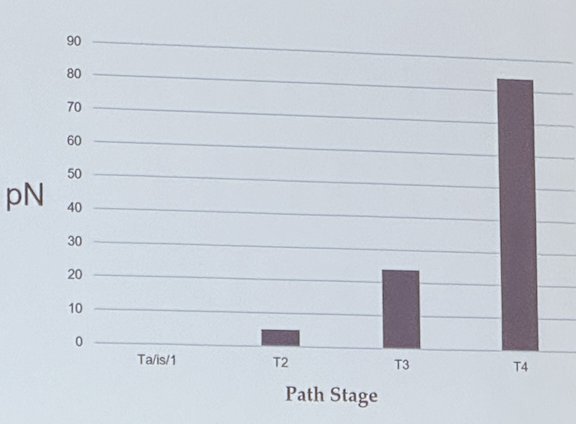
The main barrier to appropriately identifying patients with lymph node involvement is that cross-sectional imaging has very low sensitivity in detecting UTUC pN+ disease. Dr. Djaladat quoted a study by Pallouf assessing 865 patients with UTUC, of whom 750 were cN0 and 115 were cN+. Final pathology analysis showed that of the cN0 patients, 168 (22.4%) became pN+ and of the cN+ patients, only 56 (49%) ended up having pN+ disease. The sensitivity was 25%, and specificity was 91% (AUC 0.58) for lymph node involvement assessment using cross-sectional imaging.1
However, there have been some predictors associated with lymph node involvement in UTUC. Dr. Djaladat presented Venkat’s et al. nomogram for predicting lymph node metastasis in UTUC. This study identifies that having high-grade disease (OR 3.76), LVI present or unknown (OR 14.2), cN+ status (OR ranging from 18.7 to 22.6 according to clinical cN stage), and tumor size >5cm (OR 1.36) were associated with an increased risk of lymph node involvement in multivariable analysis.2
He then emphasizes that accurate lymph node staging is critical for patient selection for adjuvant systemic therapy. He briefly presented the design of the POUT trial,3 which evaluated adjuvant chemotherapy with Gemcitabine + platinum versus surveillance. He highlighted how in their subgroup analysis of patients with N+ disease, there was no apparent added benefit in disease-free survival or overall survival. This was largely because this subgroup comprised a very small number of patients (n=24), with only 14 disease-free survival and death events registered. (Forest plot shown below)
Similarly, in the CheckMate 274 trial, which compared adjuvant Nivolumab versus placebo for muscle-invasive urothelial carcinoma, the subgroup analysis of patients with renal pelvis or ureteral UTUC did not show an added benefit with Nivolumab (HR 1.23, 95% CI 0.67-2.23). This lack of benefit could possibly be related to understaging (pNx) due to the absence of systematic lymph node dissection in all patients in the trial.![]()
Dr. Djaladat emphasized that there is uncertainty regarding whether LND in UTUC is associated with improved oncological outcomes. He presented multiple retrospective studies with contradictory results, highlighting the lack of clear evidence for an oncological benefit of LND. He went on to say that there are no randomized controlled trials (RCTs) evaluating the effect of LND on oncological outcomes following nephroureterectomy or segmental ureterectomy. The best evidence supporting LND comes from meta-analyses by guideline committees, although the European Association of Urology (EAU) guideline panel found a meta-analysis for LND in UTUC not feasible and performed a narrative assessment instead. The EAU guideline states that LND appears to be unnecessary in cases of TaT1 UTUC because of the low risk of lymph node metastasis.
Dr. Djaladat showcased a systematic review by the American Urological Association (AUA) UTUC guidelines that favored LND in UTUC (HR 0.58, 95% CI 0.4-0.83). However, the AUA guidelines recommend that limited evidence exists to support a beneficial role for LND at the time of nephroureterectomy or segmental/distal ureterectomy, and no RCT has compared LND versus no LND with respect to the impact on oncologic outcomes. Clinicians may consider LND at the time of nephroureterectomy or ureterectomy according to clinically or radiographically suspicious regional lymphadenopathy or other intraoperative findings suggesting more advanced disease for which nodal staging may be warranted.5
Lastly, he briefly delved into patterns of lymphatic metastasis and anatomical templates for UTUC, showcasing the templates proposed by expert panels and guidelines, as shown below.
Dr. Djaladat underscored important key takeaway messages:
- There is no level 1 evidence to support the role of LND in UTUC
- There is still uncertainty what is the value of LND, is it diagnostic/prognostic or therapeutic?
- LND allows for more accurate risk stratification, counseling, and recruitment for adjuvant therapy or clinical trials.
- The offset is that there is some discrepancy in clinical grading/staging/risk stratification prior to surgery to appropriately select patients for LND
- He said it is good citizenship to stick to guidelines (AUA/SUO)
Presented by: Hooman Djaladat MD, MS, Urologic Oncologist, University of Southern California (USC), Los Angeles, CA
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
References:
- Pallauf M, D'Andrea D, König F, Laukhtina E, Yanagisawa T, Rouprêt M, Daneshmand S, Djaladat H, Ghoreifi A, Soria F, Fujita K, Boorjian SA, Potretzke AM, Mari A, Roumiguié M, Antonelli A, Bianchi A, Khene ZE, Sfakianos JP, Jamil M, Boormans JL, Raman JD, Grossmann NC, Breda A, Heidenreich A, Del Giudice F, Singla N, Shariat SF, Pradere B. Diagnostic Accuracy of Clinical Lymph Node Staging for Upper Tract Urothelial Cancer Patients: A Multicenter, Retrospective, Observational Study. J Urol. 2023 Mar;209(3):515-524.
- Venkat S, Khan AI, Lewicki PJ, Borregales L, Scherr DS. Novel nomograms to predict muscle invasion and lymph node metastasis in upper tract urothelial carcinoma. Urol Oncol. 2022 Mar;40(3):108.e11-108.e17. doi: 10.1016/j.urolonc.2021.11.027. Epub 2022 Jan 13. PMID: 35034804.
- Birtle AJ, Jones R, Chester J, Lewis R, Biscombe K, Johnson M, Blacker A, Bryan RT, Catto JWF, Choudhury A, Das P, Jagdev S, Powles T, Wagstaff J, Cheung KC, Cafferty F, Hall E. Improved Disease-Free Survival With Adjuvant Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Cancer: Final Results of the POUT Trial. J Clin Oncol. 2024 May 1;42(13):1466-1471.
- Bajorin, Dean F., et al. First results from the phase 3 CheckMate 274 trial of adjuvant nivolumab vs placebo in patients who underwent radical surgery for high-risk muscle-invasive urothelial carcinoma (MIUC). (2021): 391-391.
- Coleman J, et al. Non-Metastatic Upper Tract Urothelial Carcinoma - American Urological Association." AuaNet.Org, 2021,


