(UroToday.com) In anticipation of the 2021 American Urological Association (AUA) Annual Meeting which is being held, in a delayed fashion, in September, the AUA hosted a “May Kick-Off Weekend” which highlighted a variety of important topics in both benign urology and urologic oncology. Sunday morning, Costas Lallas led a course entitled “Chemotherapy and Immunotherapy for the Urologist and Advanced Practice Provider” along with faculty Edouard Trabulsi and Anne Calveresi.
In the third talk in this course, Dr. Lallas presented on renal cancer. He began by giving an overview of the epidemiology of the disease, emphasized that the majority are renal cell carcinoma and of the clear cell type. Reflecting in part the preponderance of diagnosis of early stage disease, he showed that kidney cancer is among the top 10 most common tumor types for both men and women, but not among the top 10 causes of cancer-related death for either gender.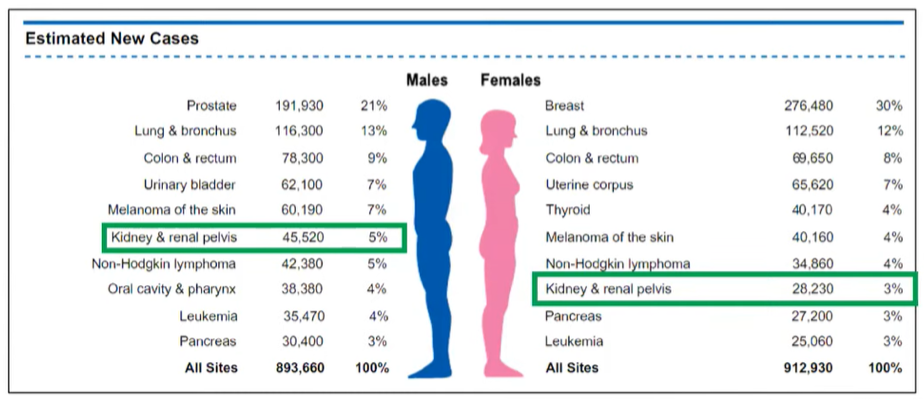
He then gave a brief overview of renal tumor histology emphasizing that clear cell renal cell carcinoma (RCC) comprises the vast majority of these, and an even higher proportion of patients with metastatic disease.

He further emphasized that, despite stage migration due to increasing incidental diagnosis as a result of abdominal imaging, a significant proportion of patients present with metastatic disease.
Moving to the discussion of systemic therapy for advanced disease, Dr. Lallas emphasized that renal cell carcinoma is minimally responsive to chemotherapy. After more than 80 clinical trials involving more than 4000 patients, the objective response rate is only 6% and, as a result, cytotoxic chemotherapy is rarely used in kidney cancer, apart from limited circumstances in those with non-clear cell histologies where limited data support the use of carboplatin and other regimes.
As a result, other systemic approaches have been used. Dr. Lallas then discussed evidence for an immunologic treatment paradigm in RCC. Early evidence of this came from observations of spontaneous remissions, the regression of metastases after treatment of the primary lesion (the abscopal effect), and the increased risk of cancer in immunodeficient states.
As a result of this, cytokine therapy was among the first successful systemic therapeutic approaches in advanced kidney cancer. The so-called cytokine era lasted from the early 1980s until 2005 and, while treatment algorithms from the time seem somewhat complex, only two options were available: interferon-alpha (INF-α) and interleukin-2 (IL-2).
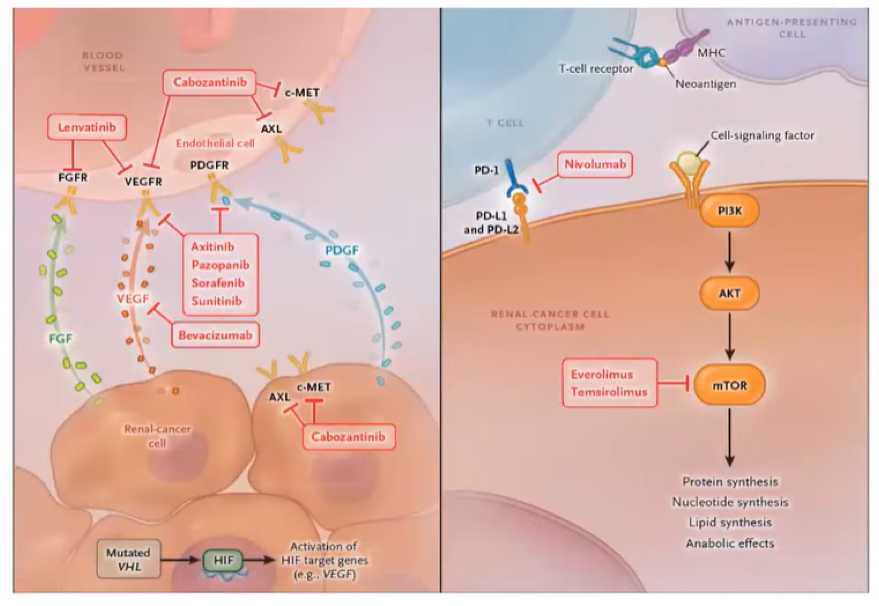
Following the cytokine era, we moved into the targeted therapy era. This dramatic evolution in the treatment of advanced kidney cancer was predicated on the biologic understanding of the VHL gene and its biologic functions, including accumulation of the transcription factor HIF and overexpression of angiogenic and proliferative genes including VEGF. Targeting this pathway with one of nine different targeted therapies showed significant survival benefits.
Perhaps the most seminal work leading to the dawn of the targeted therapy era was published in 2007, comparing first line treatment with sunitinib versus INF-α. Objective response rates were significantly higher with sunitinib at 31% and median progression-free survival was improved by 6 months.
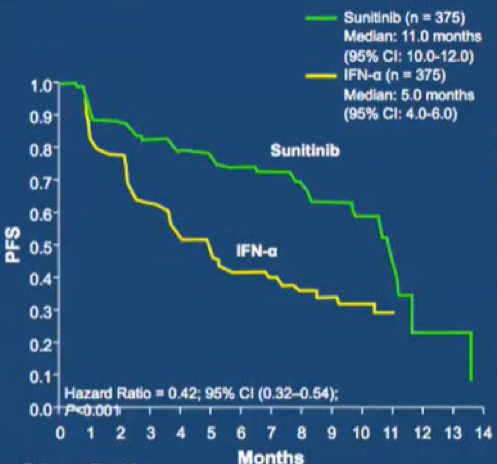
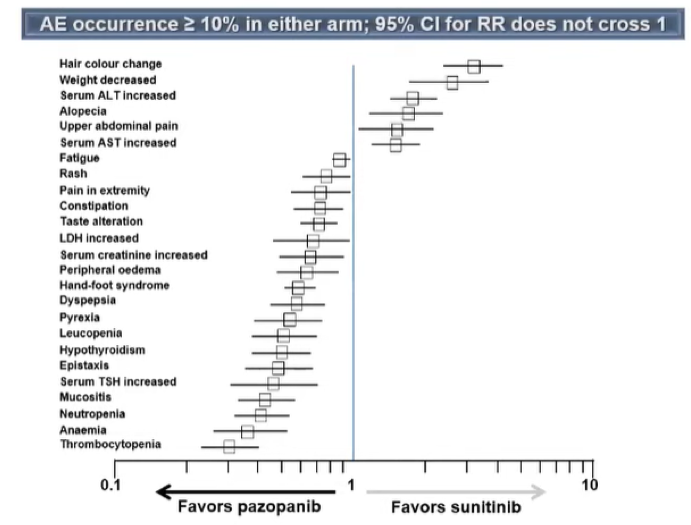
However, toxicity was higher in patients who received sunitinib as compared to those who received INF-α, particularly with respect to grade 3 and 4 diarrhea, hypertension, and hand-foot syndrome. The TARGET trial showed a similar benefit of sorafenib compared to placebo in the first line setting. In the coming years, many trials as highlighted in the table below would examine a number of different targeted agents, all acting as tyrosine kinase inhibitors or mTOR inhibitors.
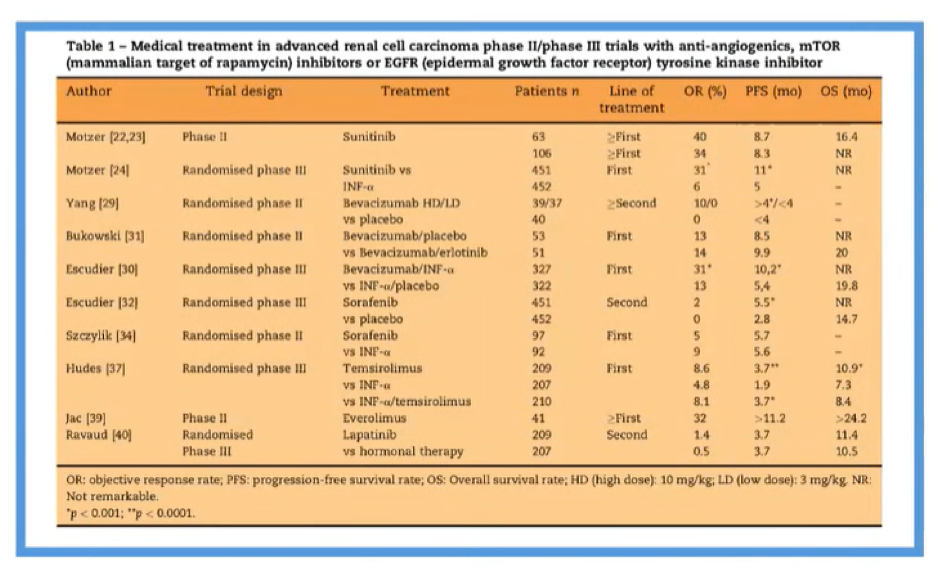
Nearly all of these compared the novel agent to IFN-α. However, in 2013, the COMPARZ trial provided an active comparison of two different targeted therapies: pazopanib was compared with sunitinib with a non-inferiority design with the goal of proving oncologic equivalence but better tolerability for pazopanib.
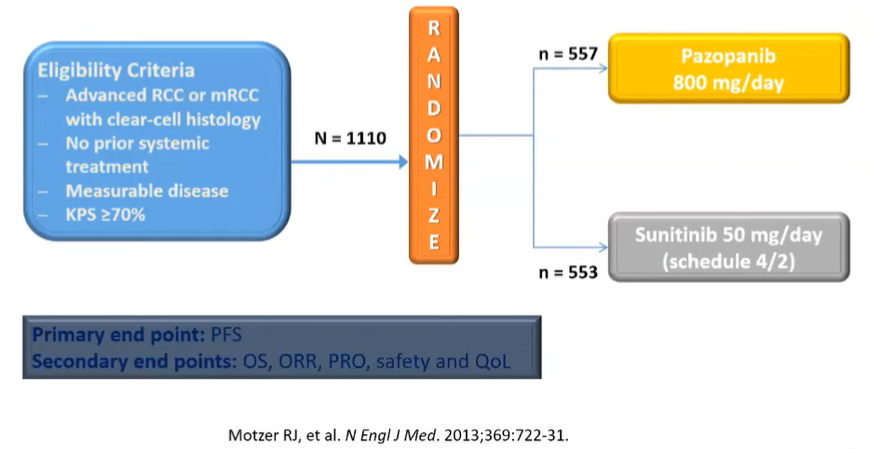
Indeed, the authors did show the non-inferiority of pazopanib with respect to both progression-free survival and overall survival. In addition, the relative risk of treatment-related adverse events was lower for patients receiving pazopanib and it quickly became a recommended first-line agent.
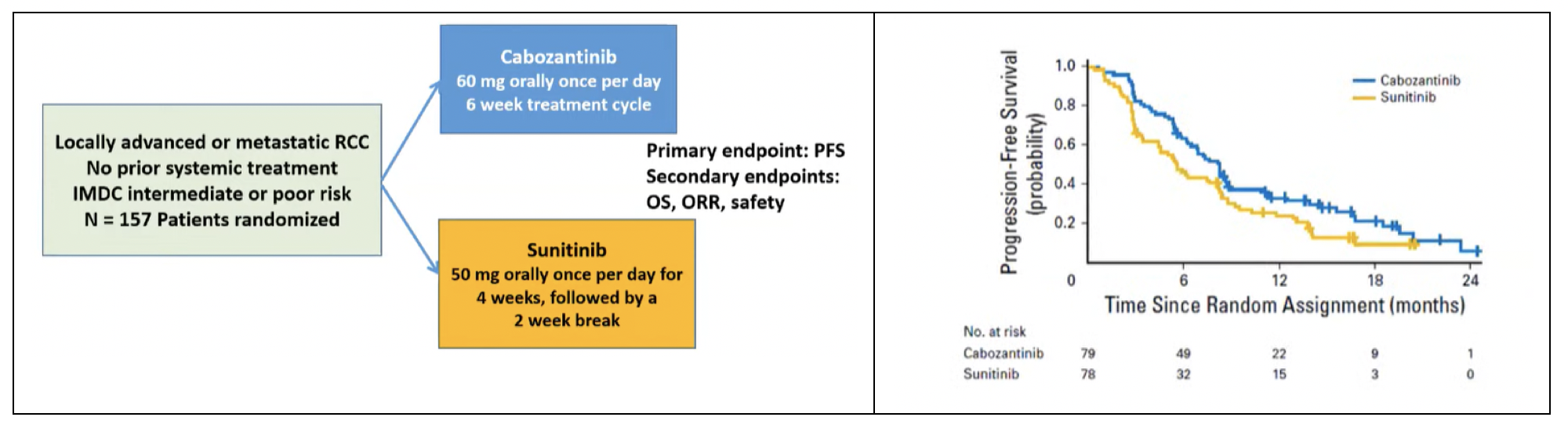
Advances in the treatment of advanced kidney cancer continued with refinements in targeted therapy. The next agent to be examined and prove benefit was cabozantinib, a multi-kinase inhibitor which targets MET and AXL in addition to VEGF. In the phase II CABOSUN trial, cabozantinib was compared with sunitinib as first-line treatment of patients with intermediate and poor-risk patients. This study demonstrated improved progression-free survival for those receiving cabozantinib (median 8.2 months) compared to suntinib (median 5.6 months), with generally comparable toxicity. This was, notably, the first trial to demonstrate superiority over sunitinib and led to cabozantinib’s approval.
The next key advance in the care of patients with advanced kidney cancer was the beginning of the checkpoint inhibitor era. This began in the second-line setting with the comparison of nivolumab and everolimus for patients who had progressed following prior anti-angiogenic therapy (CheckMate-025). Not only did nivolumab have better efficacy (hazard ratio for overall survival 0.73, 98.5% CI 0.57 to 0.93), it was better tolerated with lower rates of grade 3 or 4 treatment-related adverse events (19% versus 37%).
Following these positive results in the second-line space, a number of trials assessed immune checkpoint inhibitor combinations in the first line setting. The first of these to be published showing benefit was CheckMate-214 which compared nivolumab (an anti-PD-1 agent) and ipilimumab (an anti-CTLA-4 agent) with sunitinib in previously untreated patients with advanced ccRCC. Over a median follow-up of 25 months, both overall survival and progression-free survival were higher among patients who received the immune checkpoint inhibitor combination therapy. However, this was notably affected by risk stratification: while the combination approach was superior in patients with intermediate and poor risk disease, objective response rates and progression-free survival were better with sunitinib among patients with favourable risk disease. Grade 3 or 4 treatment-related adverse events occurred in 46% of patients receiving nivolumab and ipilimumab and 63% of patients receiving sunitinib. These data led to the approval of the combination of nivolumab and ipilimumab for patients with intermediate or poor risk disease. It does warrant mention, however, that 75-80% of all patients with metastatic RCC will have intermediate or poor risk disease, according to the IMDC risk criteria.
Given the established success of VEGF-targeted tyrosine kinase inhibitor therapy, as well as the new data support immune checkpoint inhibition, combinations of these approaches were then explored in a number of trials. Keynote-426 provided a phase III comparison of pembrolizumab and axitinib versus sunitinib in patients with previously untreated advanced kidney cancer, demonstrating a significant improvement in both progression-free survival and overall survival which was robust across all IMDC risk groups and independent of PD-L1 expression. This led to the approval of this combination for patients, independent of risk group. Notably, toxicity was somewhat more common among patients in the combination arm of this trial, compared to those who received sunitinib. Published in the same issue of the New England Journal of Medicine as Keynote-426, JAVELIN Renal 101 compared avelumab and axitinib versus sunitinib in the same patient population. Notably, this study was designed with stratification according to PD-L1 expression with a focus on the PD-L1 positive population as the primary endpoint though an unselected group was examined as a secondary outcome. A benefit in progression-free survival was seen with combination therapy in both the PD-L1 positive group and the overall study cohort. However, at the time of publication, overall survival data were immature with median follow-up of less than 12 months. In contrast with Keynote-426, toxicity with the combination therapy approach in JAVELIN Renal 101 was comparable to that seen with sunitinib. These data led to the approval of both pembrolizumab and axitinib and of avelumab and axitinib for first-line therapy.
Dr. Lallas then briefly discussed the IMmotion151 trial which examined atezolizumab and bevacizumab versus sunitinib. While the study met its primary endpoint, demonstrated improved progression-free survival in patients with PD-L1 positive disease, the interim analysis of overall survival data was immature and many suggest that the viability of this approach depends on longer term outcomes.
He then discussed the more recently published CheckMate-9ER trial which compared the combination of nivolumab and cabozantinib versus sunitinib. Across IMDC groups and PD-L1 status, this study demonstrated the superiority of the combination of nivolumab and cabozantinib for progression-free survival and overall survival. Thus, these data formed the basis of the recent approval of this combination.
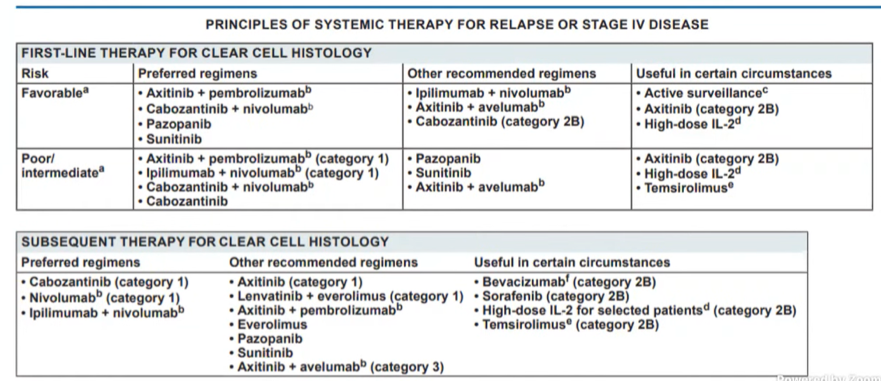
As a result of these rapidly emerging data, as of February 2021, the NCCN Kidney Cancer guidelines now recommend a host of treatment options.
Notably, while relevant, Dr. Lalla did not discuss the recently published CLEAR trial which was also presented at ASCO-GU in February 2021.
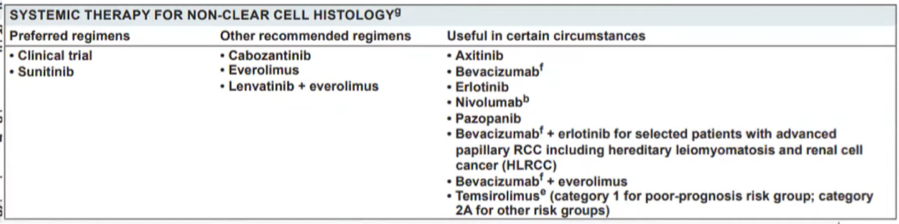
Thus far, the data presented has focused on treatment options for patients with ccRCC. Dr. Lallas then presented the NCCN guideline recommendations for non-clear cell histology. While the recent SWOG 1500 trial demonstrated the superiority of cabozantinib to sunitinib, sunitinib or clinical trials remain the preferred regimes.
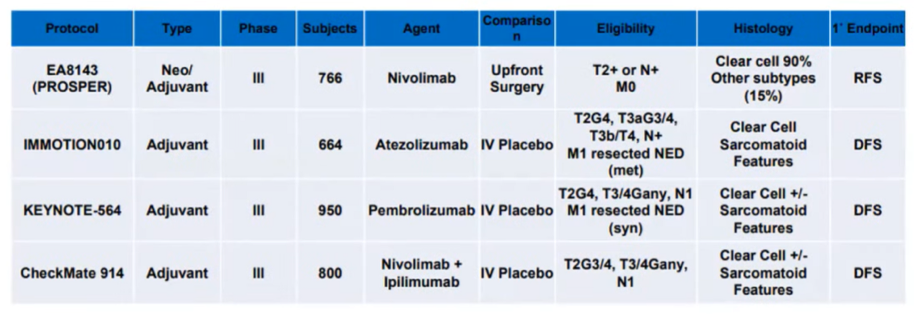
Dr. Lallas then briefly discussed adjuvant therapy in RCC. Apart from somewhat controversial results of S-TRAC, all studies of anti-VEGF therapy and mTOR inhibitors in this setting have failed to demonstrate benefit. However, ongoing and imminently reporting trials are examining the role of immunotherapy in this setting.
In closing, he briefly discussed cytoreductive nephrectomy. This was established as dogma on the basis of two randomized controlled trials which were subsequently meta-analyzed. This pooled analysis demonstrated approximately 6 months improvements in median overall survival.
However, he noted that only 5.6% of patients in these trials failed to receive systemic therapy following nephrectomy. In contrast, observational studies in the targeted therapy era suggested that upfront cytoreductive nephrectomy may limit receipt of systemic therapy and thus worsen outcomes. This finding was recapitulated in the CARMENA trial of cytoreductive nephrectomy followed by sunitinib versus sunitinib alone: 17.7% of patients in the surgical arm never received sunitinib. This trial failed to demonstrate a benefit to cytoreductive nephrectomy. Addressing the question of cytoreductive nephrectomy somewhat differently, the SURTIME trial compared immediate versus deferred nephrectomy for patients receiving sunitinib, finding improved outcomes for patients undergoing the deferred approach.
Presented by: Costas Lallas, MD, Professor, Vice-Chair, Academic Affairs Associate Director Urology Residency Program, Jefferson Health
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter during the AUA2021 May Kick-off Weekend May 21-23


