(UroToday.com) In anticipation of the 2021 American Urological Association Annual Meeting which is being held, in a delayed fashion, in September, the AUA is hosting both a “May Kick-Off Weekend” and “Summer School” which are highlighting a variety of important topics in both benign urology and urologic oncology. On June 15th, 2021, Dr. Marc Bjurlin, Dr. Thomas Hope, and Dr. Michael Gorin discussed the role of advanced imaging, with a focus on positron emission tomography (PET), in modern urologic oncology practice.
As the final talk in this session, Dr. Hope presented the role of prostate-specific membrane antigen (PSMA)-PET in prostate cancer. PSMA is a transmembrane protein that is heavily overexpressed in prostate cancer, though it is not specific to prostate cancer. He emphasized that there are many agents which can be used for PSMA-based imaging, though they share a urea motif. While this family has similarities, there are differences in biodistribution with differences in clearance. Regardless of the specific tracer used, these are efficacious for the detection of prostate cancer.
Dr. Hope presented the results of a trial at UCSF examining PSMA-11 compared to the gold standard of prostatectomy and lymphadenectomy histopathology. This approach demonstrated high specificity (95%) though sensitivity was relatively limited (40%). A similar study based out of both UCSF and UCLA among patients with biochemical recurrence demonstrated a high positive predictive value of PSMA-PET. Most importantly, Dr. Hope emphasized that these studies are relatively easy to read and, as a result, there is high inter-reader concordance. As a result of these data, the FDA approved 68Ga-PSMA-11 for men with suspected metastasis who are candidates for initial definitive therapy and though with suspected recurrence based on elevated PSA levels, based on UCSF and UCLA in December 2020.
While this approval of 68Ga-PSMA represents important progress in the care of patients with prostate cancer, there are many limitations. Potentially the greatest of these is that gallium-68 has only a 68-minute half-life which limits its ability to be distributed. Further, there is a relatively limited ability to produce 68Ga as only 2-3 doses can be made per synthetic run. Thus, this emphasizes the importance of other agents. This is addressed with the recent approval of 18F-DCFPyL. Dr. Hope emphasized that the results of the OSPREY and CONDOR trials of this radiotracer are nearly identical to those from the assessment of 68Ga from UCSF and UCLA.

Therefore, Dr. Hope concluded that the data support that these agents are essentially interchangeable. He then addressed, on this basis, a comparison of results of PSMA-based imaging (using 68Ga-PSMA) to fluciclovine PET/CT. Dr. Hope emphasized that PSMA has a much better tumor to background ratio increasing the ability to identify lesions. Based on a prospective study from UCLA, he emphasized that fluciclovine essentially was only able to identify localized disease whereas 68Ga-PSMA based imaging detected disease at a much higher rate, including nodal and distant metastatic disease.
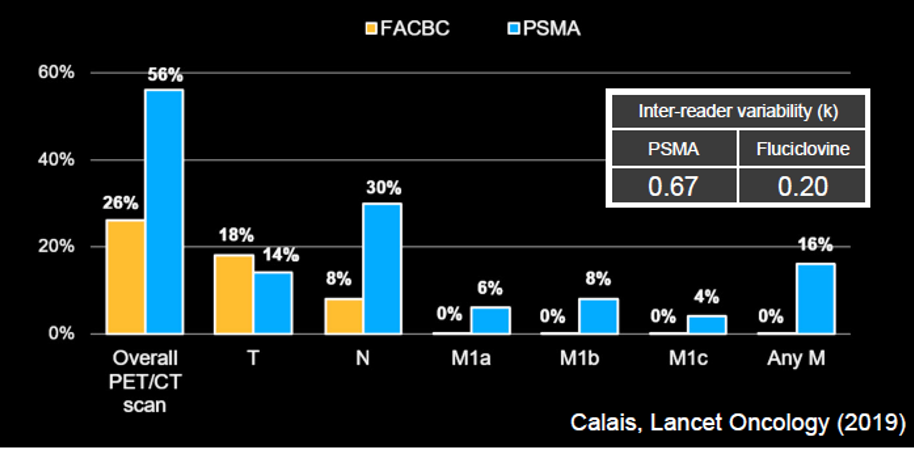
Further, fluciclovine had much worse inter-reader reliability (Kappa 0.20 vs 0.67).
He then transitioned to a discussion of insurance coverage. As both 68Ga-PSMA-11 and DCFPyL have been FDA approved, they should be covered by Medicare for both their approved indications. However, in terms of private insurance, he emphasized the need for updates to the NCCN guidelines to support the role of PSMA-PET for reimbursement.
He further emphasized that there are numerous ongoing trials assessing different PSMA-based radiotracers including PSMA-1007, rh-PSMA-7, and PSMA-617.
Dr. Hope emphasized that, while there are strengths to PSMA-based imaging, there are many reasons for false positives. These include a number of benign lesions including rib lesions, pre-sacral ganglia, dorsal root ganglia, hemangiomas, Paget’s disease, and fibrous dysplasia. However, other tumors including hepatocellular carcinoma, thyroid cancer, and lung cancer (adenocarcinoma) may also result in false-positive disease. Additionally, because of urinary excretion, careful interpretation is necessary when examining local recurrent disease and pelvic nodal disease which is adjacent to the ureter.
In terms of the impact of PSMA imaging, Dr. Hope emphasized that the negative predictive value is not sufficiently high to exclude pelvic lymphadenectomy in patients with high-risk prostate cancer. However, routine use of PSMA-PET/CT prior to radiotherapy may change the treatment plan in more than half of patients. Thus, the effect of pre-treatment PSMA on treatment decisions may differ based on the initial treatment planned.
Following radical prostatectomy, PSMA-PET/CT can assist with the localization of the disease. Work from UCSF suggested that 30% of patients with the biochemically recurrent disease may have disease outside the standard radiotherapy field based on PSMA imaging. Incorporating the knowledge gained from molecularly targeted imaging may results in changes to salvage radiotherapy regimes and patients’ outcomes, as was recently demonstrated in the recently published EMPIRE-1 trial, utilizing fluciclovine. A comparable concept to EMPIRE-1 is being tested using PSMA-based imaging. Based on the comparative data cited before, we may expect that the use of PSMA-PET would have an even greater benefit than fluciclovine.
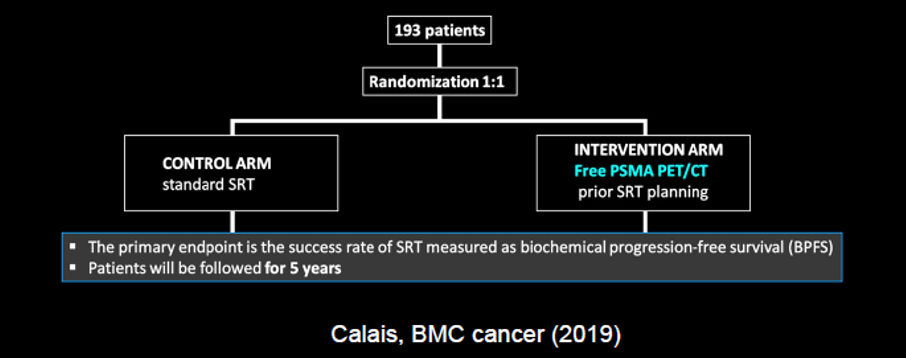
While, in biochemically recurrent disease, the majority of patients have a chance in management, to date, it is unclear how these changes in management translate to clinical outcomes.
Finally, Dr. Hope transitioned to a discussion of theranostics, the use of a compound for both diagnostics and therapeutics. The TheraP trial was the first randomized comparison establishing that theranostic treatment with 177Lu-PSMA was associated with improved clinical outcomes compared to the accepted standard of care at that point in the disease trajectory, cabazitaxel.
Dr. Hope then highlighted the many ongoing trials of PSMA-targeted radionuclide therapy, including both industry-sponsored and academic trials.
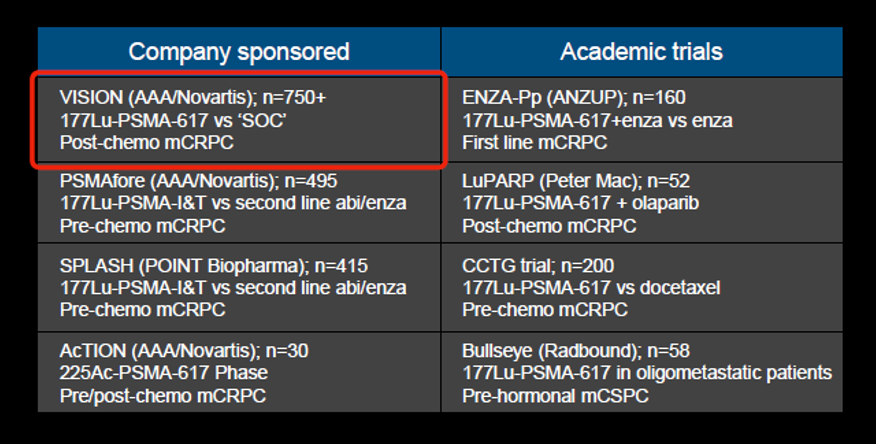
One of these, the VISION trial, was reported recently at ASCO 2021, demonstrating improvements in both overall and progression-free survival for patients receiving 177Lu-PSMA.
Dr. Hope concluded that PSMA-PET is superior to existing radiotracers for the detection of metastatic prostate cancer. Based on recent FDA approvals, he suggested that PSMA-PET should be widely available in the United States by the end of 2021.
Presenter: Thomas Hope, MD, Assistant Professor in Abdominal Imaging and Nuclear Medicine, Department of Radiology, University of California- San Francisco
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter, during the AUA2021 May Kick-off Weekend May 21-23.


