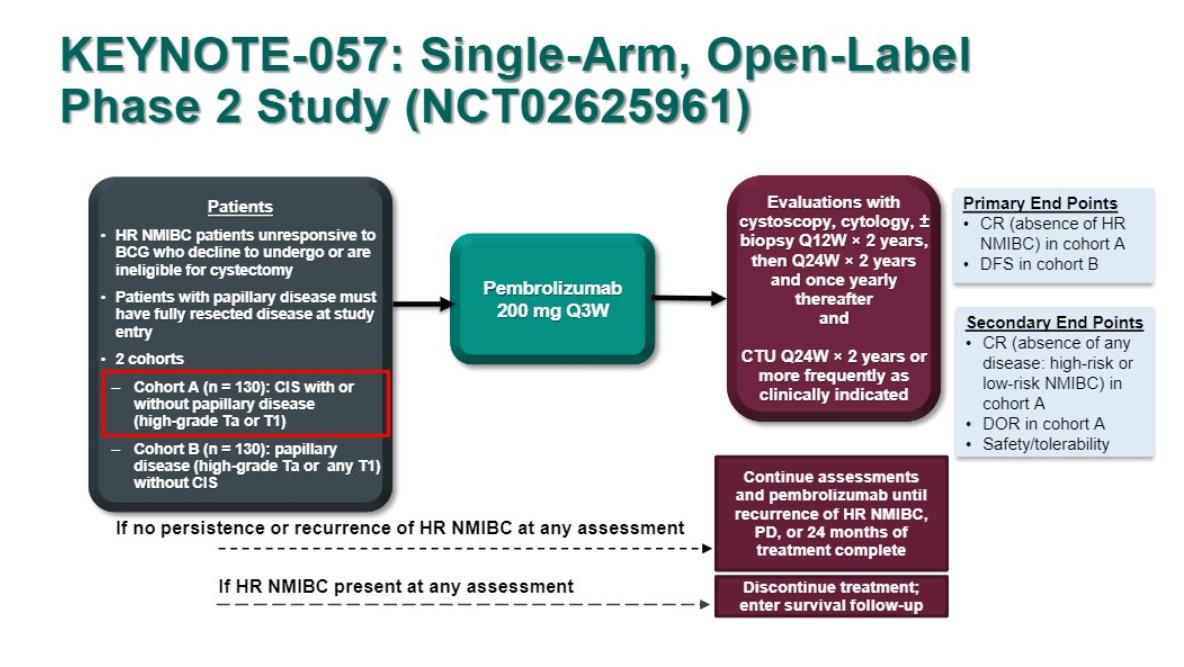
The median number of prior BCG installations was 12 with 2/3 patients had CIS alone. The complete response (CR) rate at 3mo was 40.6% which exceeded the 20% success criterion for the primary hypothesis test. Duration of CR: out of 96 patients, 39 achieved CR at first disease assessment with median Duration of response, (DoR), 16.2 months and at 12 mo CR was 19% for all patients enrolled. Favorable toxicity profile and no major changes in quality of life were observed. The United States Federal Drug Administration's approval has redefined multidisciplinary care with co-management between urology and medical oncology will lead to improved care for our patients.
Presented by: Arjun V. Balar, MD, Medical Oncologist, Director of the genitourinary medical oncology program at NYU Langone’s Perlmutter Cancer Center, New York, New York
Written by: Stephen B. Williams, MD, Medical Director for High-Value Care; Chief of Urology, Professor, Director of Urologic Oncology, Director Urologic Research, The University of Texas Medical Branch at Galveston, TX at the 2020 Virtual Bladder Cancer Advocacy Network Think Tank 2020
Related Content:
Watch: A Debate on The Management of BCG Unresponsive - Cystectomy Ineligible Bladder Cancer Patient: Pembrolizumab Vs. Nadofaragene Firadenovec - Arjun Balar & Peter Black
Watch: Pembrolizumab in the BCG-unresponsive NMIBC Patients - Arjun Balar
Watch: Pembrolizumab in High-Risk, BCG Unresponsive Non-Muscle Invasive Bladder Cancer - Arjun Balar


